Iopamidol synthesis and preparation of iopamidol synthesis intermediate
A technology of iopamidol and intermediates is applied in the field of synthesis and the preparation of synthetic intermediates, can solve the problems of waste of reactants, difficult production and the like, and achieves the effects of less reaction time, production cost saving, and fast reaction rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0030] A kind of synthesis of iopamidol and the preparation of synthetic intermediate thereof, the synthesis of described iopamidol and the preparation technology of synthetic intermediate thereof comprise following route and step:
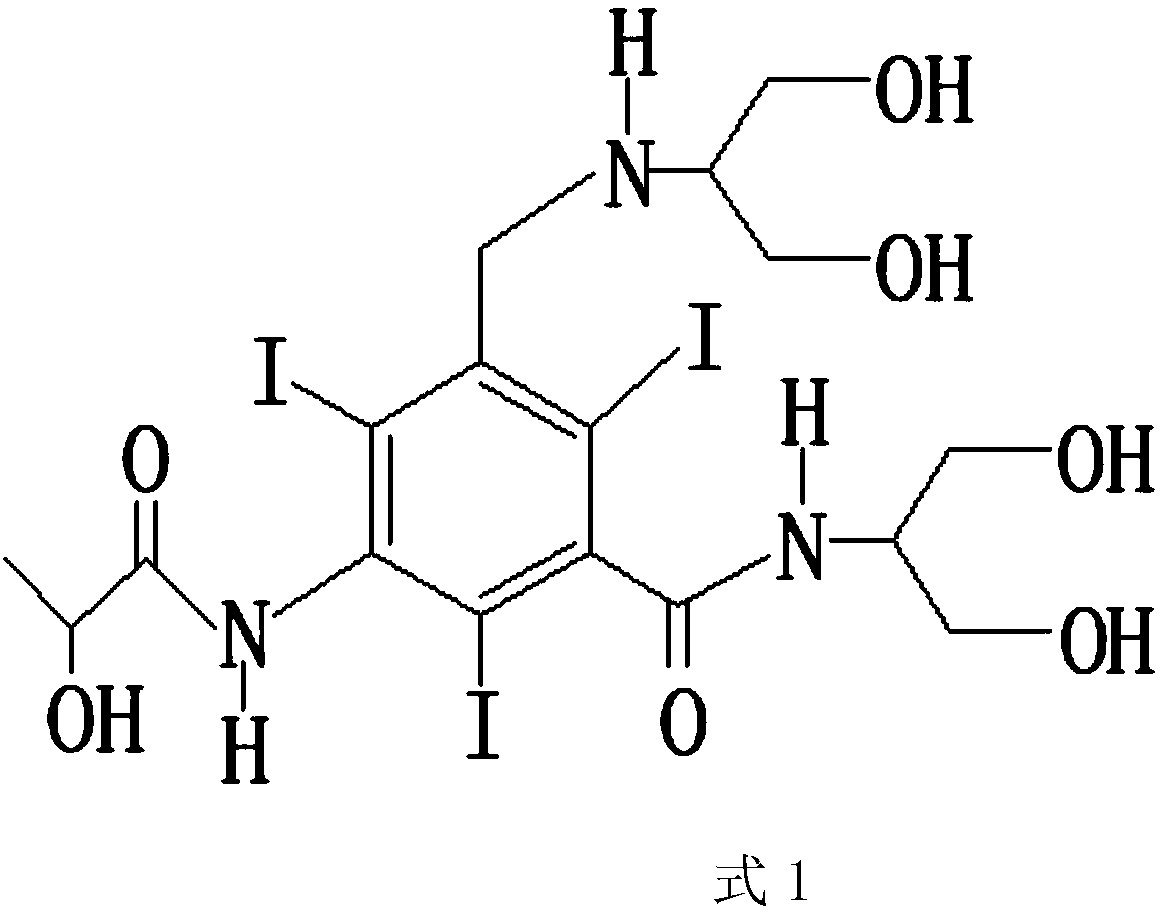
[0031] The molecular structure of described iopamidol is as shown in formula 1:
[0032]
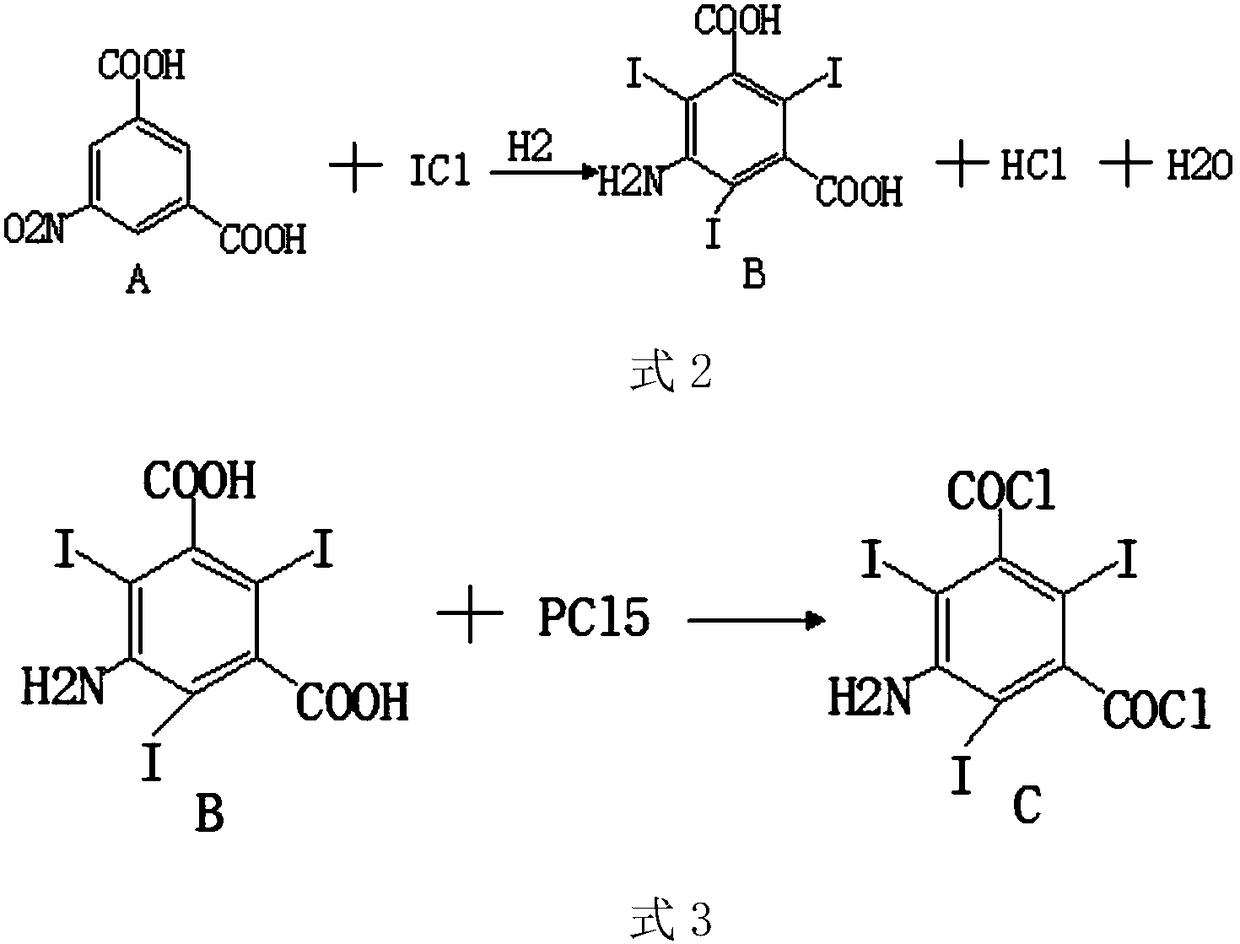
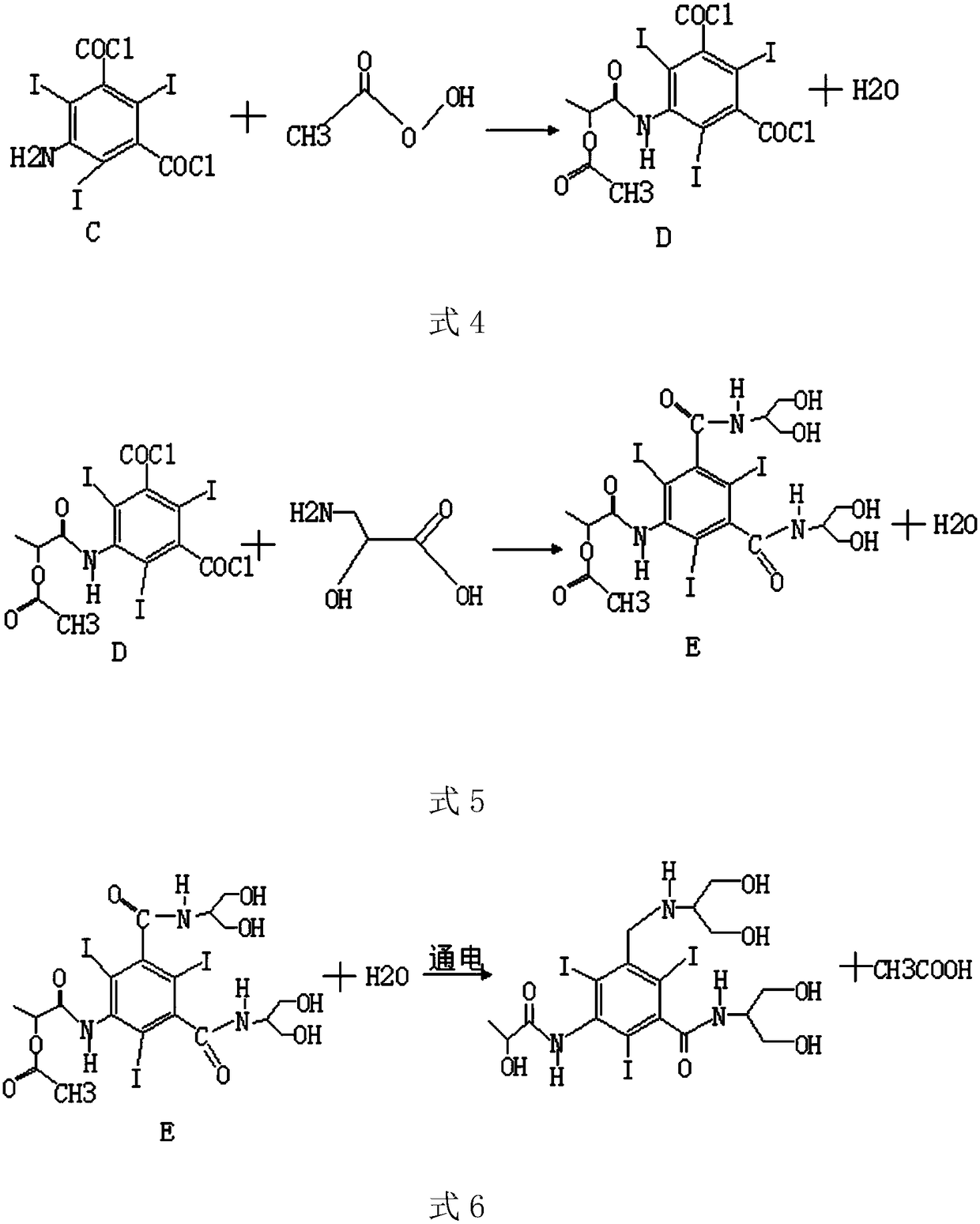
[0033] The preparation process route is as follows:
[0034]
[0035]
[0036] Step 1: Mix a certain amount of compound A and iodine chloride in a reaction vessel, add a certain amount of catalyst, and react fully to generate compound B, hydrogen chloride and water;
[0037] Step 2: Take a certain amount of compound B and phosphorus pentachloride and mix them in a reaction vessel. After fully reacting, compound C is generated;
[0038] Step 3: Mix a certain amount of compound C and acetoxy compound in a reaction vessel, add a certain amount of catalyst, and react fully to generate compound D and water;
[0039] Step 4: Mix a certain amount of comp...
Embodiment
[0043] S1: Mix 200g of compound A and 300g of iodine chloride in a container, and then continue to pour hydrogen into the container to control the intake rate of hydrogen, and control the total molar amount of hydrogen to 20 moles until the end of the reaction , the reaction temperature is controlled at about 70 degrees Celsius, and the reaction time is controlled at 5 hours. During the reaction, the conduit is connected to the exhaust port of the reaction vessel, and the other end of the conduit is connected to a sealed collection container;
[0044] S2: adding a certain amount of hydrochloric acid to the mixed solution after the reaction of compound A and iodine chloride, so that the pH value of the mixed solution is about 5;
[0045] S3: in step 1, the product generated after the reaction of compound A and iodine chloride is compound B, get 100g compound B and 150g phosphorus pentachloride and mix in a container, the reaction temperature is controlled at about 30 degrees Cel...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com