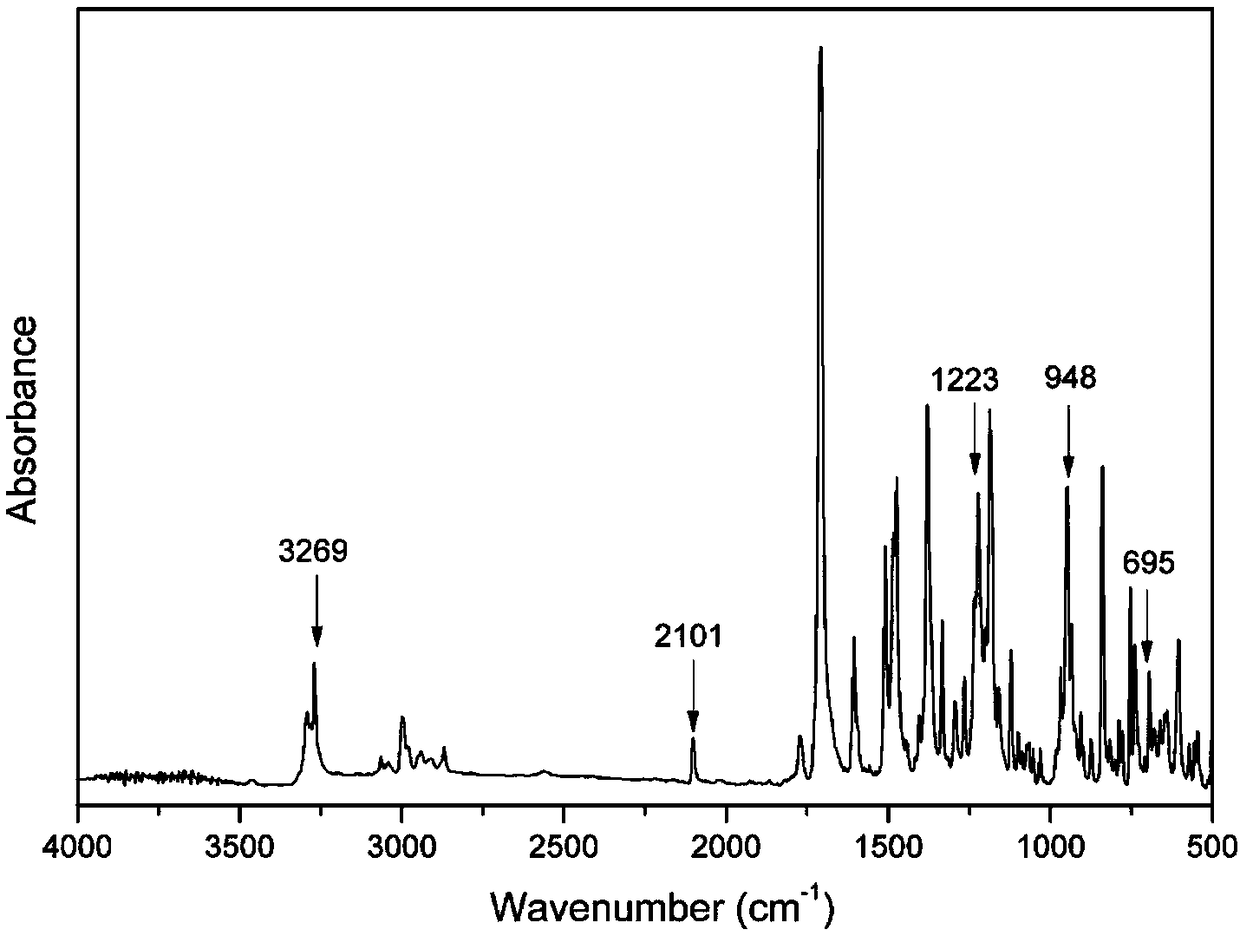
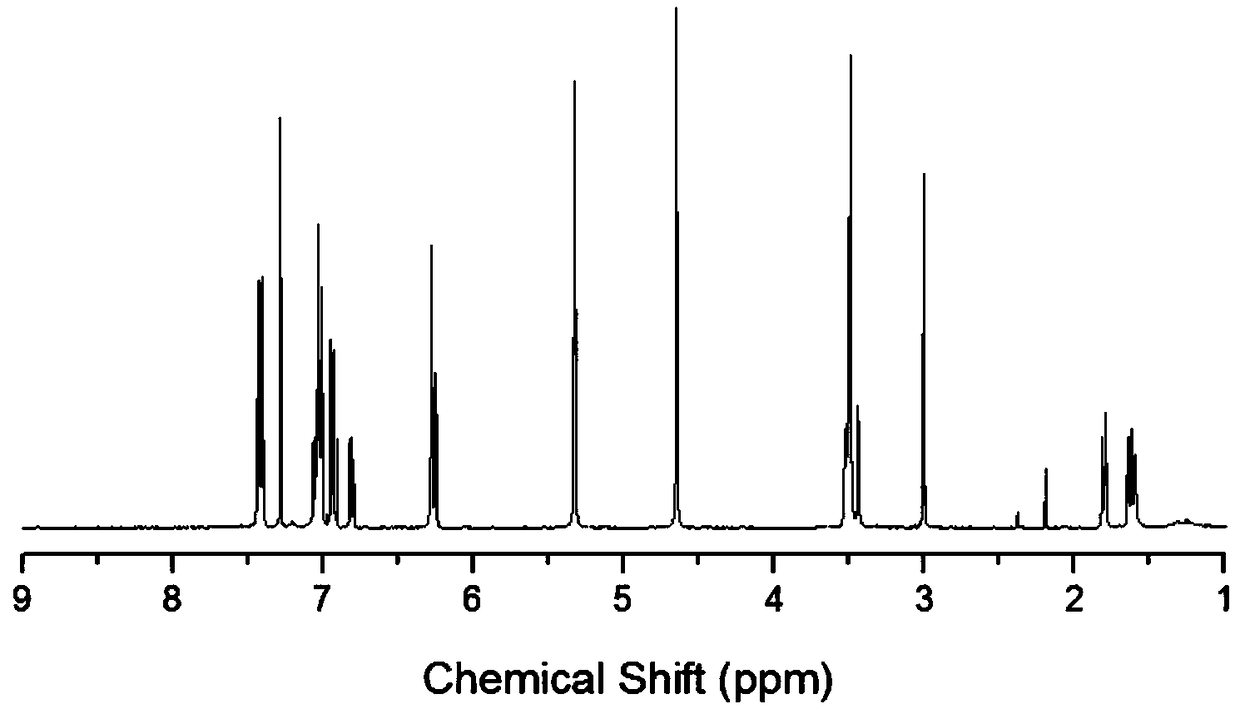
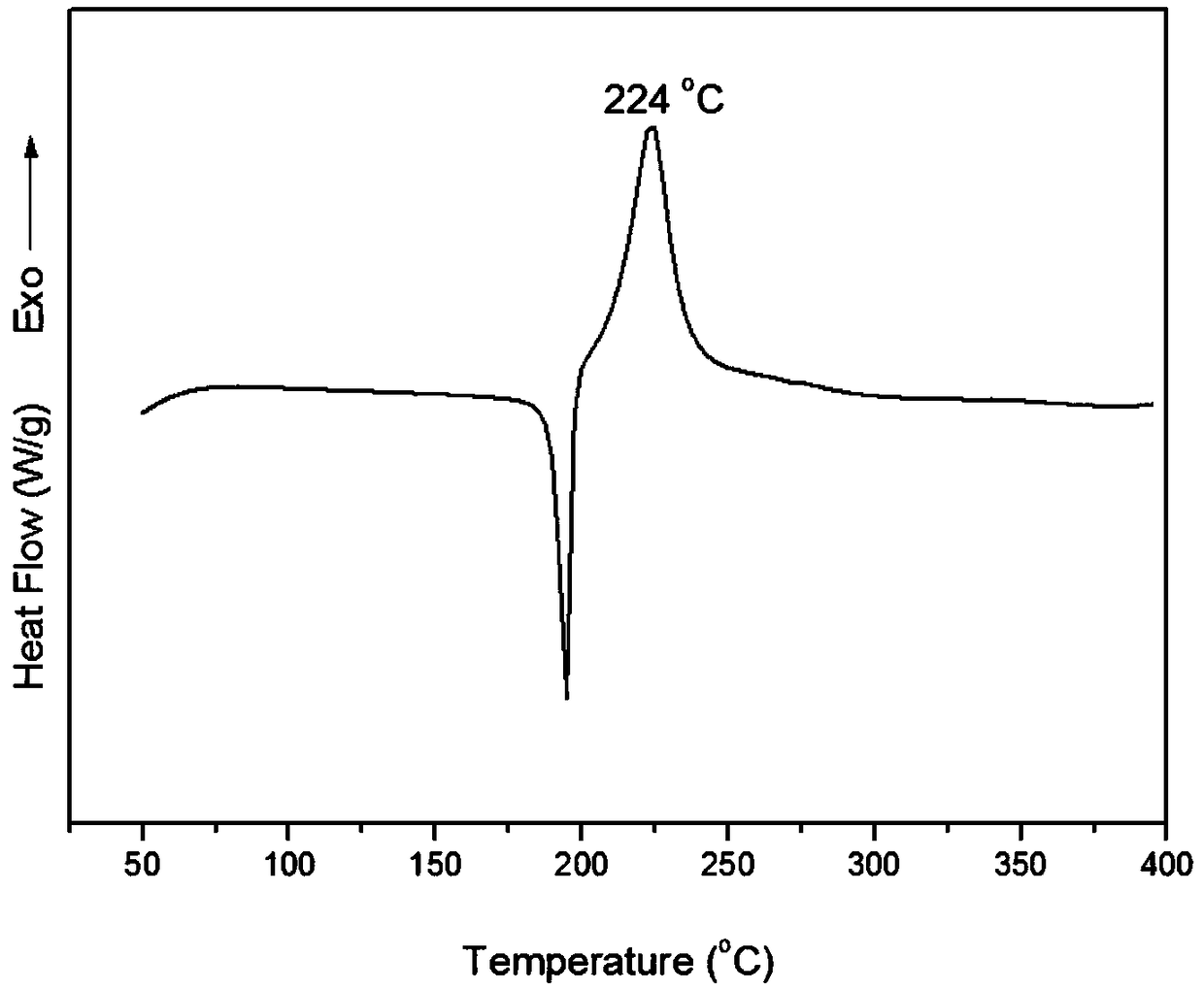
Benzoxazine monomer containing ethynyl groups and norbornene and preparation method and application of benzoxazine monomer
A technology of norbornene and benzoxazine, which is applied in the field of benzoxazine monomer and its preparation, can solve the problems of excessively high processing temperature, increase of preparation cost, high thermal curing temperature of norbornene, and reduce the processing temperature , improve thermodynamic properties, simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] The specific steps are:
[0037] (1) Norbornene dioic anhydride 1g, o-aminophenol 0.665g, 40mL glacial acetic acid (AcOH) are respectively added in the reaction vessel equipped with stirring magnet and condenser, the reaction unit is moved to the oil bath, 120 ℃ of reaction for 6 hours, after the reaction, the reaction solution was poured into 150 mL of deionized water to obtain a large amount of precipitation, after suction filtration, washed three times with a large amount of deionized water, and put into a 60 ℃ vacuum drying box to obtain the product ortho-norbornene Functionalized phenol 1.435 g, yield 92%. The reaction equation is as follows:
[0038]
[0039] (2) Weigh 1 g of ortho-norbornene functionalized phenol and 0.459 g of 4-ethynylaniline, 0.254 g of paraformaldehyde, and 40 mL of xylene prepared in step (1), respectively, and add them to a stirring magnet and a condenser. In the reaction vessel of the tube, the reaction was carried out at 120 ° C for ...
Embodiment 2
[0047] The reactant 4-ethynylaniline in the second step in Example 1 was replaced with 3-ethynylaniline, and other operation steps were the same as those in Example 1 to obtain 1.256 g of the product with a yield of 82%.
[0048] The specific chemical structure of the obtained main chain polybenzoxazine is:
[0049]
[0050] The temperature of the benzoxazole resin obtained in this example is 428° C. when the thermal weight loss is 5%, the carbon residue rate at 800° C. is 63%, and the dielectric constant of the benzoxazole resin at room temperature and 1 MHz is 2.7.
Embodiment 3
[0052] The reactant 4-ethynylaniline in the second step in Example 1 was replaced with 2-ethynylaniline, and other operation steps were the same as those in Example 1 to obtain 1.364 g of the product with a yield of 88%.
[0053] The specific chemical structure of the obtained main chain polybenzoxazine is:
[0054]
[0055] The benzoxazole resin obtained in this example has a temperature of 443° C. at a thermal weight loss of 5%, a carbon residue rate of 68% at 800° C., and a dielectric constant of 2.6 at room temperature and 1 MHz of the benzoxazole resin.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com