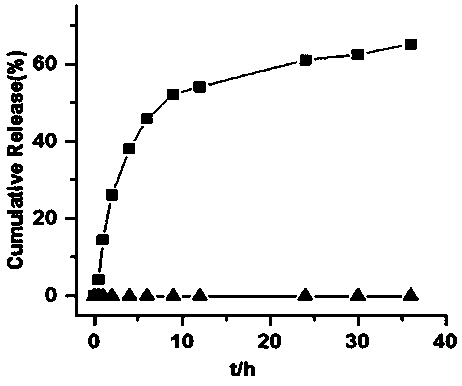
Reduction-responsive type camptothecin dimer and reduction-sensitive drug delivery system based on the camptothecin dimer
A delivery system, the technology of dihydroxyethyl disulfide, which is applied in the fields of biomedicine and nanomedicine, can solve the problems of poor water solubility of camptothecin, toxic and side effects, and low drug loading capacity, and achieves excellent anti-tumor, simple preparation, Water solubility and stability enhancement effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
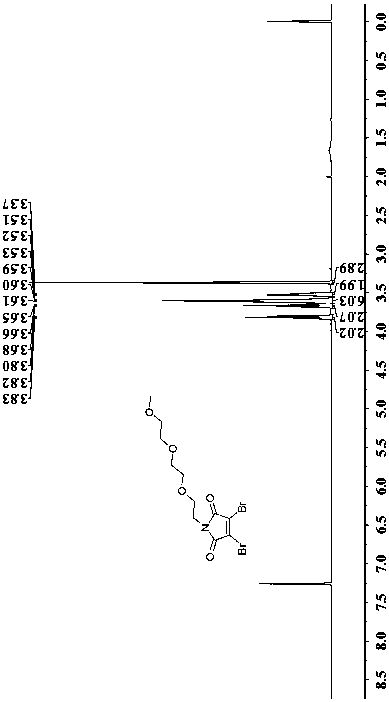
[0029] Dissolve 511 mg (1.95 mmol) of triphenylphosphine in 5 mL of anhydrous THF and stir. Add 0.38 mL (1.95 mmol) of diisopropyl azoxylene to the above solution dropwise, and stir at -78°C for 5 min. After dissolving 0.25 mL (1.56 mmol) of triethylene glycol monomethyl ether in 5 mL of anhydrous THF, it was added dropwise to the reaction solution of diisopropyl azoxylene, and stirred at -78°C for 5 min. Afterwards, 121 mg (1.37 mmol) of neopentyl alcohol was dissolved in 2 mL of anhydrous THF and added to triethylene glycol monomethyl ether, and stirred at -78°C for 5 min. Finally, 497 mg (1.95 mmol) of dibromomaleimide was added to the above reaction solution, stirred at -78°C for 10 min, then transferred to room temperature, and magnetically stirred at room temperature for 20 h. The crude product was purified by column chromatography (volume ratio EA:PA=1:10-1:4), and then separated by medium-pressure preparation to obtain 325 mg of the off-white target product, Intermedi...
Embodiment 2
[0031] Weigh 123.2mg (0.8mmol) of 2-hydroxyethyl disulfide, 323.6mg (3.2mmol) of triethylamine and 230mg (0.8mmol) of TCEP.HCl and dissolve in 50mL of anaerobic-treated methanol, and stir at room temperature for 20min. Weigh 319.1mg (0.8mmol) of the intermediate 1 prepared in the example and dissolve it in 25mL of anaerobic-treated methanol, then add it to the above reaction solution, and the color changes from colorless to turmeric. Stir magnetically overnight at room temperature. The crude product was purified by column chromatography (volume ratio methanol:dichloromethane=1:200-1:50) to obtain 243.3 mg of the target pure intermediate 2 in the form of dark yellow oil with a yield of 77%. 1 H NMR (600MHz, CDCl 3 )δ3.91–3.81(m,2H),3.72(d,2H),3.69–3.57(m,5H),3.54(s,1H),3.48–3.42(m,2H),3.38(s,2H) ,2.28–1.96(m,1H).
Embodiment 3
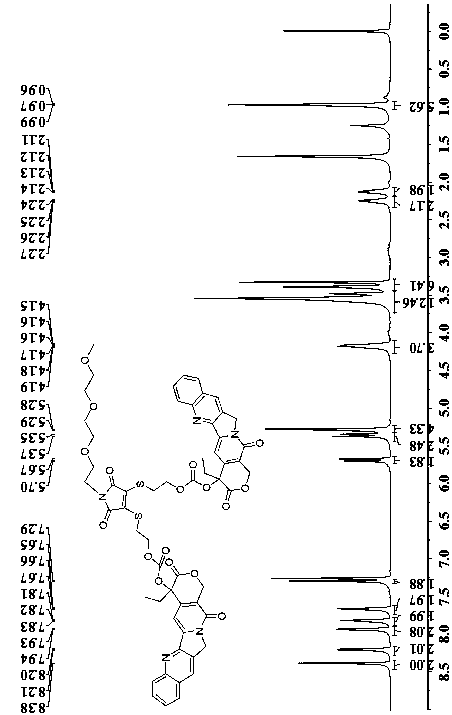
[0033] Weigh 835.4mg (2.4mmol) of CPT and dissolve it in 25mL of anhydrous dichloromethane, add 482.35mg (2.4mmol) of p-nitrophenyl chloroformate and 585.9mg (4.8mmol) of DMAP under stirring, under anaerobic conditions Stir for 4h. Then weigh 395.1mg (1.0mmol) Intermediate 2 prepared in Example 2 and dissolve it in 16mL (anhydrous THF / CH 2 Cl 2 ) solution. Magnetic stirring was carried out at room temperature for 36 h. The crude product was purified by column chromatography (volume ratio methanol:dichloromethane=1:250-1:100) to obtain 525.9 mg of the yellow-green target product camptothecin dimer 3 with a yield of 64%. 1 H NMR (600MHz, CDCl 3)δ 8.38(s,2H),8.20(d,2H),7.93(d,2H),7.82(t,2H),7.66(t,2H),7.29(s,2H),5.69(d,2H) ,5.36(d,2H),5.29(d,4H),4.17(m,4H),3.51(m,12H),3.38(m,6H),2.25(m,2H),2.12(m,2H), 0.98(t,6H). 13 CNMR (151MHz, CDCl 3 ( s), 128.58–128.04(m), 120.26(d, J=0.9Hz), 95.92(s), 78.01(s), 71.89(s), 70.53(s), 70.02(s), 67.63(d, J =11.6Hz), 67.12(s), 59.01(s), ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com