Reduction sensitive polyamino acid nano hydrogel and preparation method thereof
A nano hydrogel and polyamino acid technology, applied in the field of hydrogel, can solve the problems of complex polyester preparation process and unfavorable large-scale production, and achieve rapid release of reduction response, good biodegradability and biocompatibility , improve the effect of curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
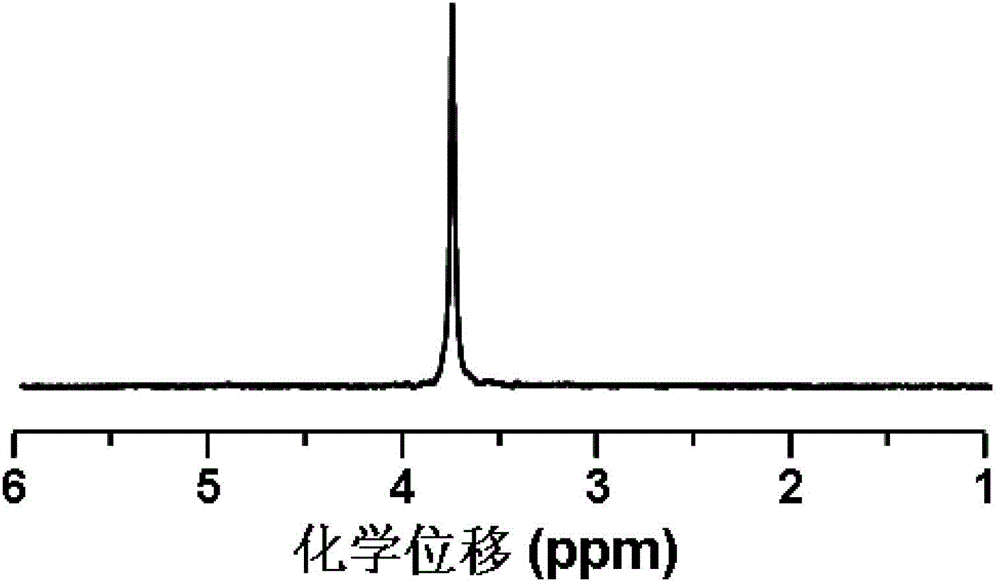
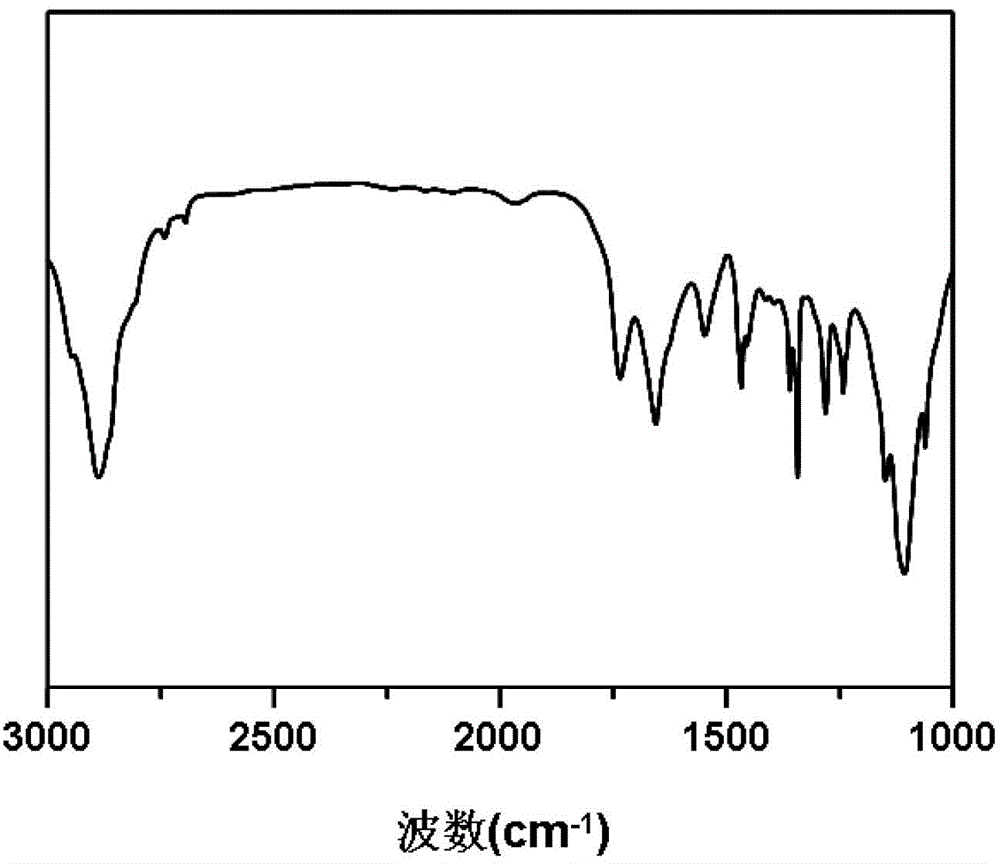
[0061] Add 1.1 g of aminated polyethylene glycol monomethyl ether with a number-average molecular weight of 550 to the dry reaction flask, remove water with 100 mL of anhydrous toluene at 130 ° C for 2 h, and vacuum dry the remaining Toluene; the obtained solid was dissolved in 10 mL of dry N,N-dimethylformamide to obtain the first solution; 2.06 g of γ-propynyl-L-glutamate-N-carboxylic acid anhydride was dissolved in 30 mL of dry In N,N-dimethylformamide, the second solution was obtained; in a nitrogen atmosphere, the first solution was mixed with the second solution, stirred under nitrogen protection conditions, and reacted at 40°C for 24h; after the reaction, reduce N,N-dimethylformamide was dried under pressure, and then the obtained solid was dissolved in chloroform, then settled with ether, filtered by suction, and dried to obtain polyethylene glycol monomethyl ether-poly(γ-propyne base-L-glutamate) block copolymer;
[0062] Dissolve 0.04 g of the obtained polyethylene ...
Embodiment 2
[0065] Add 1 g of aminated polyethylene glycol monomethyl ether with a number-average molecular weight of 2000 to the dry reaction flask, and remove the water by azeotroping with 100 mL of anhydrous toluene at 130°C for 2 h, then vacuum the remaining toluene to dryness ; The resulting solid was dissolved in 10 mL of dry N,N-dimethylformamide to obtain a first solution; 1.26 g of γ-propynyl-L-glutamate-N-carboxylic acid anhydride was dissolved in 20 mL of dry In N,N-dimethylformamide, the second solution was obtained; in a nitrogen atmosphere, the first solution was mixed with the second solution, stirred under nitrogen protection conditions, and reacted at 20°C for 72h; after the reaction, the pressure was reduced Drain N,N-dimethylformamide, then dissolve the obtained solid in chloroform, settle with ether, filter with suction, and dry to obtain polyethylene glycol monomethyl ether-poly(γ-propynyl -L-glutamate) block copolymer;
[0066] Dissolve 0.04 g of the obtained polyet...
Embodiment 3
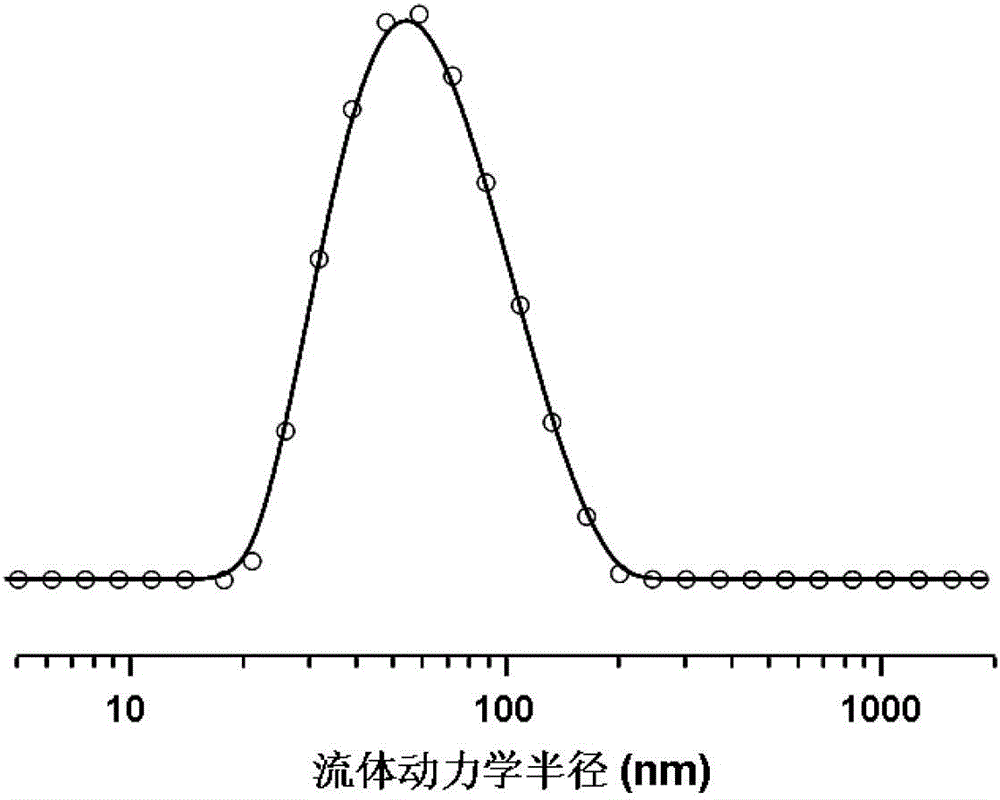
[0069] Dissolve 0.04 g of the polyethylene glycol monomethyl ether-poly(γ-propynyl-L-glutamate) block copolymer obtained in Example 2 in 10 mL of N,N-dimethylformamide, and then Slowly add it dropwise into 20mL of ultrapure water, and after stirring for two hours, remove the organic solvent by dialysis to obtain a micellar solution of the polymer, add 0.01g of cross-linking agent to the micellar solution, and then blow it with nitrogen Soak for 30 minutes, add 0.003g CuSO 4 ·5H 2 0, after bubbling for 5 minutes, add 0.01g sodium ascorbate, seal, under nitrogen atmosphere, 25 ℃ of reaction 24h, after reaction finishes, reaction solution is added in the dialysis bag, with distilled water dialyzing for three days, freeze-drying then, That is, the polyamino acid nanohydrogel with a cross-linking degree of 50% is obtained.
[0070] The polyamino acid nano-hydrogel that embodiment 3 obtains is carried out in vitro simulated release experiment, and the result shows, the release of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com