Uncaria-containing brain-targeting nano preparation and preparation method thereof
A nano-preparation, a technology containing urticine, applied in the directions of nanotechnology, nanotechnology, nanomedicine, etc., can solve the problems of improving the bioavailability of uricine, lack of slow-release and controlled-release effects, etc., and achieve good encapsulation performance. , high encapsulation rate, overcoming the effect of low solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] The synthetic route of the nuclear material 60%-γ-PFGA is as follows:
[0046]
[0047] Proceed as follows:
[0048] In step (1), 4-methyl-5-hydroxyethylthiazole (35.19mmol) and methyl iodide (106.02mmol) were reacted in an autoclave at 60°C for 3h, the reactant was transferred to a round-bottom flask with an appropriate amount of methanol, and evaporated under reduced pressure. Dry solvent, wash with appropriate amount of ether to obtain tan 3,4-dimethyl-5-(2-hydroxyethyl)iodothiazole;
[0049] In step (2), 1-bromopropane (49.83mmol) was dissolved in 30mL of ethanol containing 0.2mL of PEG400, and 10mL of 5H containing 0.2mL of PEG400 was added dropwise 2 O·Na 2 S 2 O 3 (45.21 mmol) aqueous solution, refluxed at 69 °C for 7 h, evaporated to dryness under reduced pressure, and vacuum dried to obtain white crystalline powder propyl pente salt;
[0050] Step (3), take 3,4-dimethyl-5-(2-hydroxyethyl) iodothiazole (21.1 mmol) and dissolve it in 16 mL of water, add N...
Embodiment 2
[0060] Synthesis of shell material PLA-SS-PEG-A2:
[0061]
[0062] Take PLA-SS-PEG-MAL (the molecular weight of polylactic acid PLA is 2000Da, and the molecular weight of polyethylene glycol PEG is 2000Da) 8.322mg, Angiopep-2-Cys(A2-C) 5.28mg (PLA-SS-PEG-MAL and Angiopep-2-Cys in a molar ratio of 1:1.1), in a PBS buffer of pH 7.4, at room temperature, protected from light and anaerobic reaction for 3h; using a dialysis bag with a molecular weight cut-off of 3500Da, placed in pure water for 24h dialysis to remove Free A2-C and buffer salts were used to prepare PLA-SS-PEG-A2.
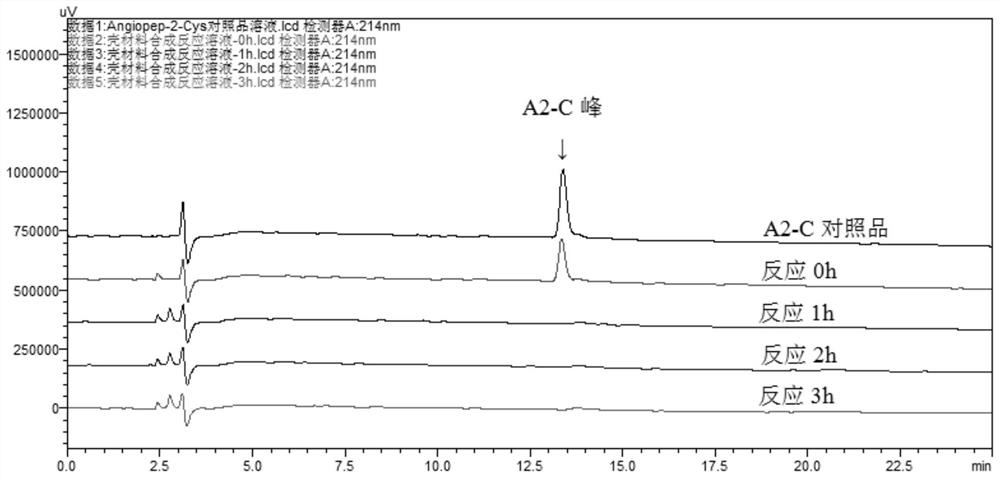
[0063] PLA-SS-PEG-A2 1 H-NMR (CDCl 3 ) spectrum shows that the chemical shift of 6.72 ppm is the characteristic peak of maleimide (MAL) group in the raw material PLA-SS-PEG-MAL, while in PLA-SS-PEG-A2 1 The characteristic peaks of MAL disappeared in the H-NMR spectrum, indicating that the MAL group of PLA-SS-PEG-MAL was coupled with the thiol group of A2-C. HPLC chart ( figure 1 ) also shows: the...
Embodiment 3
[0065] Preparation of brain-targeted nanoformulations co-delivered with rhynchophylline and Tariquidar:
[0066]Step (1), preparation of organic phase: take 10 mg of 60%-γ-PFGA (Example 1), add 0.5 mL of DMSO, stir overnight to dissolve, and obtain a 60%-γ-PFGA solution with a concentration of 20 mg / mL; 1 mg of cymenine and 0.5 mg of Tariquidar were added to the 60%-γ-PFGA solution, and stirred for 1 h to dissolve urticine and Tariquidar to obtain a 60%-γ-PFGA solution containing uricine and Tariquidar as the organic phase;
[0067] Step (2), the preparation of water phase: take 2mg PLA-SS-PEG-A2 (Example 2), 8mg E-TPGS, add 1mL of saturated urticine aqueous solution, stir and dissolve to obtain 1mL PLA-SS-PEG-A2 Aqueous phase with a concentration of 2 mg / mL;
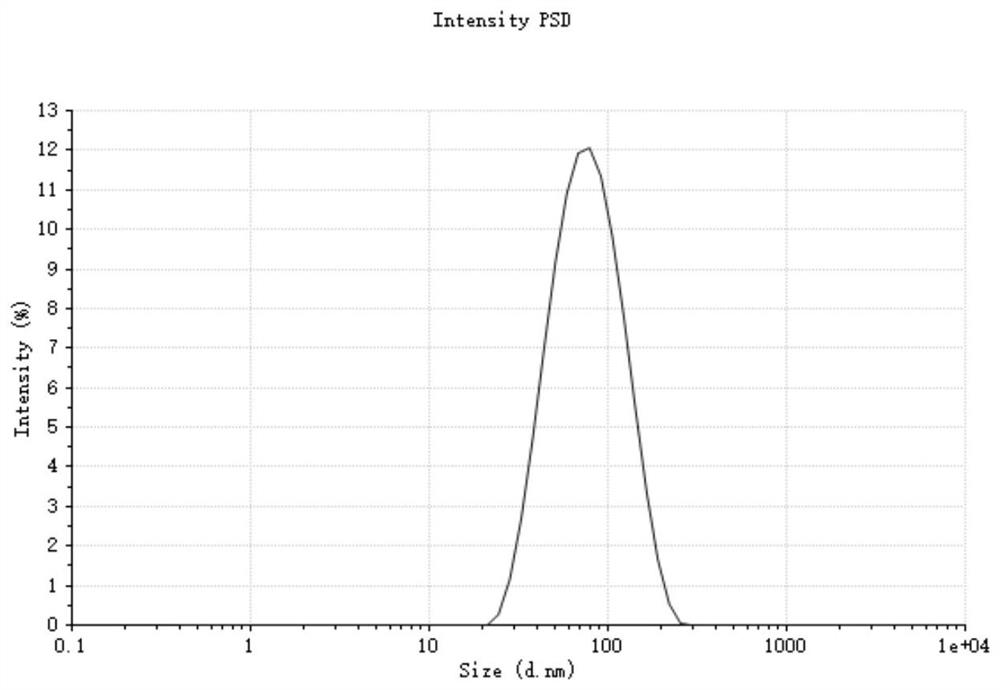
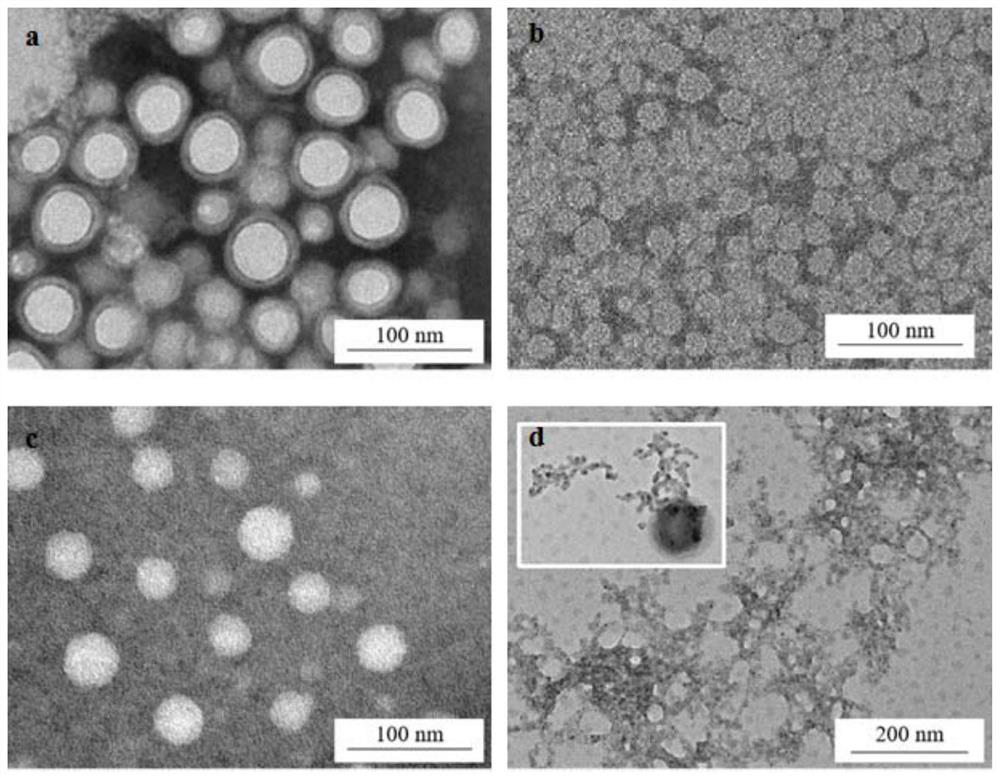
[0068] Step (3), preparation of nano-preparation by nano-precipitation method: take 1 mL of aqueous phase, heat to 65°C, add 100 μL of organic phase dropwise to the aqueous phase while stirring, stir for 1 h, and slowl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| degree of grafting | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com