Divalent metal-base MIL-53 microporous material, and preparation method and application thereof
A microporous material and metal technology, applied in the field of +2-valent metal MIL-53 microporous material and its preparation, can solve the problems of poor adsorption selectivity and weak binding force, and achieve simple steps, good stability, and adsorption. good performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] A preparation method based on +2 valent metal MIL-53 microporous material, the method is as follows:
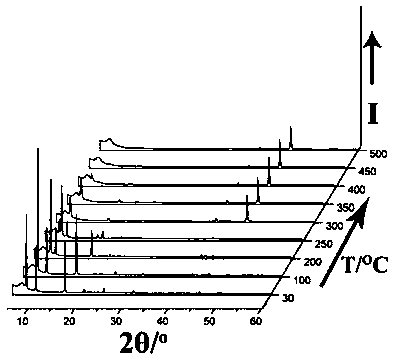
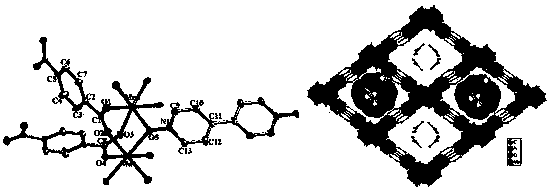
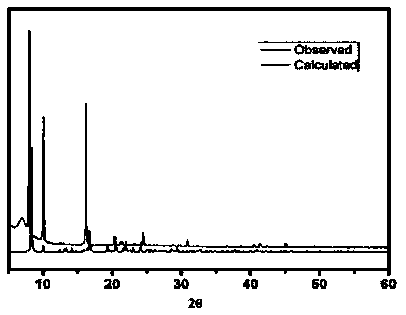
[0035] Mn(NO 3 ) 2 solution (0.3 mmol, 0.0861g), H 2 BDC (0.1mmol, 0.0224g), bpdo (0.1mmol, 0.0188g) and DMF / H 2 O (3.0 ml: 1.0 ml) was placed in a small 25.0 ml beaker, and the resulting mixture was stirred at room temperature for one hour, and the resulting solution was transferred to a 17.0 ml stainless steel reaction vessel with a polytetrafluoroethylene liner, at 120 ° C for spontaneous pressure After heating for 10 hours under certain conditions, the reaction system was slowly cooled to room temperature to obtain red rod-shaped crystals and a very small amount of colorless block-shaped crystals. After the product was filtered and dried naturally, the colorless crystals could be removed manually. Finally, put the colorless solid product into a round-bottomed flask connected with an oil pump to evacuate, and heat in an oil bath at 150° C. for 8 hours to prepar...
Embodiment 2
[0037] A preparation method based on +2 valent metal MIL-53 microporous material, the method is as follows:
[0038] Mn(NO 3 ) 2 solution (0.1 mmol, 0.0287g), H 2 BDC (0.2 mmol, 0.0448g), bpdo (0.2mmol, 0.0376 g) and DMF / H 2 O (3.0 ml: 1.0 ml) was placed in a small 25.0 ml beaker, and the resulting mixture was stirred at room temperature for one hour, and the resulting solution was transferred to a 17.0 ml stainless steel reaction vessel with a polytetrafluoroethylene liner, at 120 ° C for spontaneous pressure After heating for 10 hours under certain conditions, the reaction system was slowly cooled to room temperature to obtain red rod-shaped crystals and a very small amount of colorless block-shaped crystals. After the product was filtered and dried naturally, the colorless crystals could be removed manually. Finally, put the colorless solid product into a round-bottomed flask connected with an oil pump to evacuate, and heat in an oil bath at 150° C. for 8 hours to prep...
Embodiment 3
[0040] A preparation method based on +2 valent metal MIL-53 microporous material, the method is as follows:
[0041] Mn(NO 3 ) 2 solution (0.5 mmol, 0.1435g), H 2 BDC (0.25mmol, 0.0560g), bpdo (0.25mmol, 0.0470g) and DMF / H 2 O (3.0 ml: 1.0 ml) was placed in a small 25.0 ml beaker, and the resulting mixture was stirred at room temperature for one hour, and the resulting solution was transferred to a 17.0 ml stainless steel reaction vessel with a polytetrafluoroethylene liner, at 120 ° C for spontaneous pressure After heating for 10 hours under certain conditions, the reaction system was slowly cooled to room temperature to obtain red rod-shaped crystals and a very small amount of colorless block-shaped crystals. After the product was filtered and dried naturally, the colorless crystals could be removed manually. Finally, put the colorless solid product into a round-bottomed flask connected with an oil pump to evacuate, and heat in an oil bath at 150° C. for 8 hours to prep...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com