Application of lobetyolin similar compound in preparing drug for treating arrhythmia
A technology of Codonopsis glucoside and compound is applied in the application field of Codonopsis glucoside-like compound in the preparation of drugs for treating arrhythmia, and can solve the problems of unclear material basis of pharmacological activity, and unrevealed mechanism of action of a single effective chemical component in Codonopsis pilosula. , to achieve broad market application prospects, simple and reliable operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The extraction and separation method of embodiment 1 styoside analogue compound (compound Lobeyolinin):
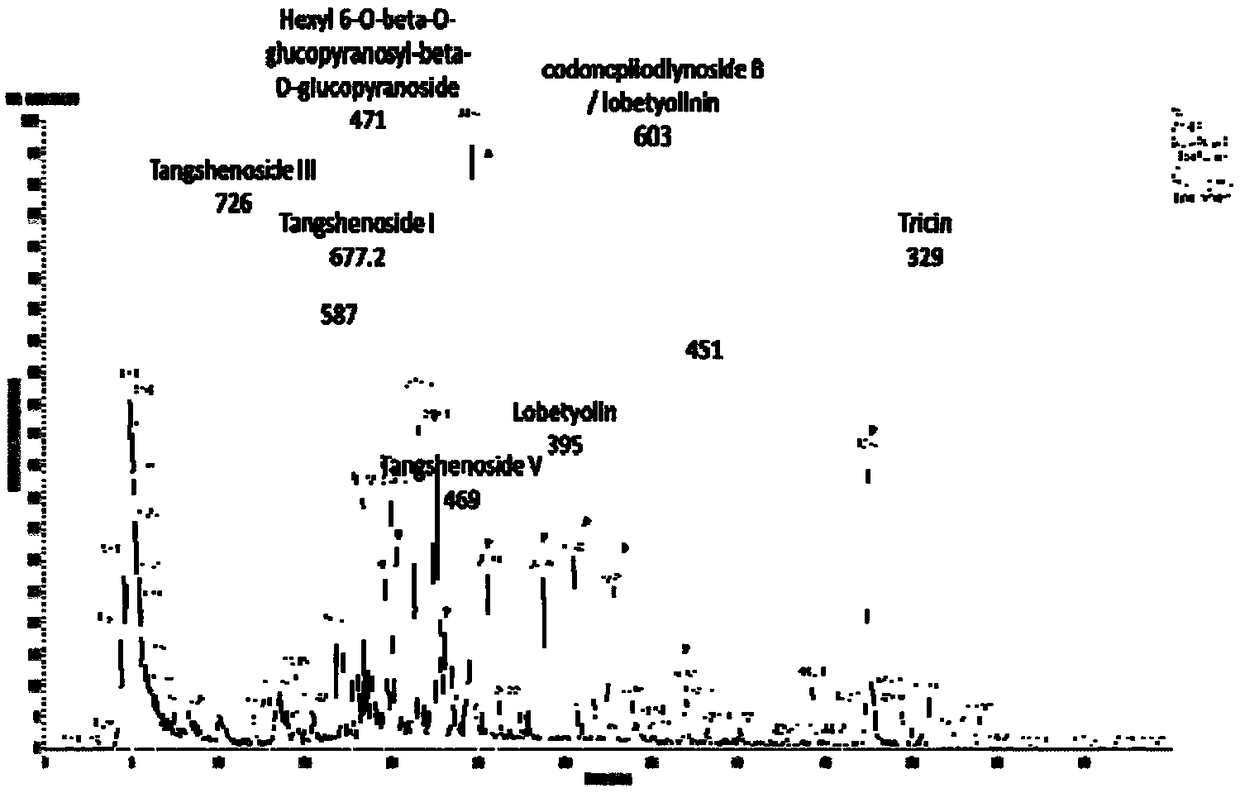
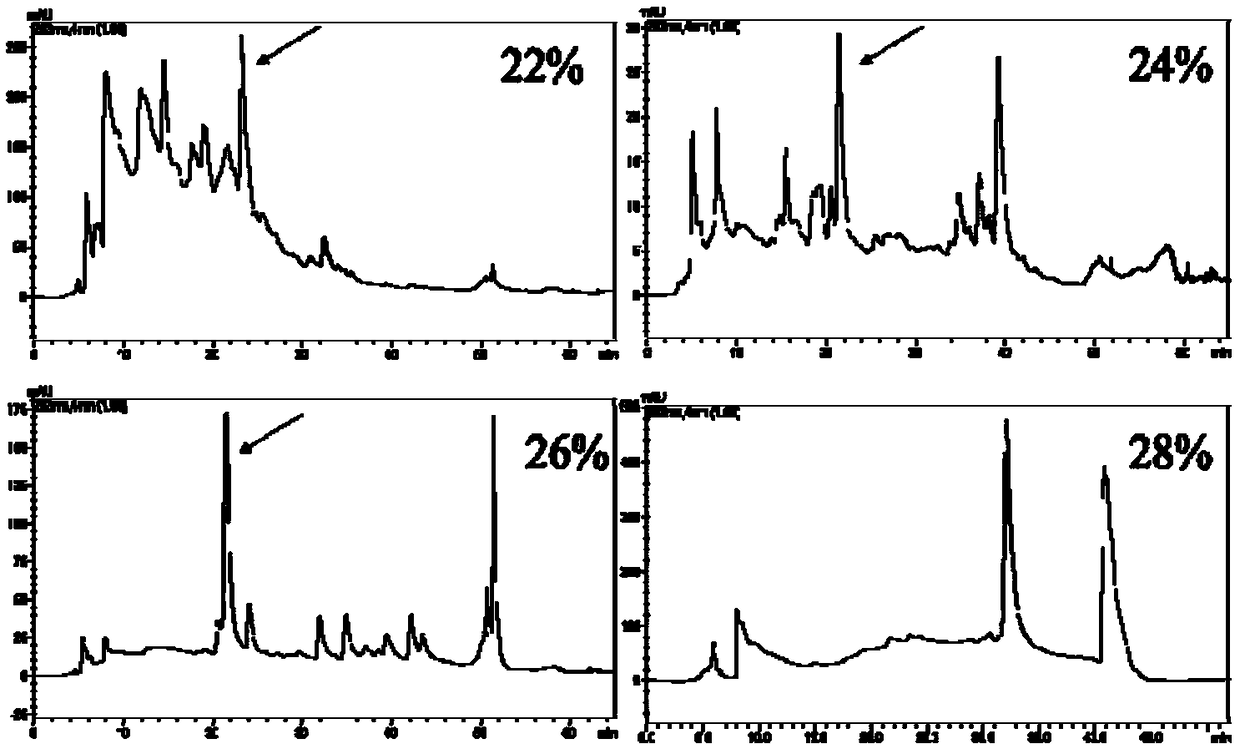
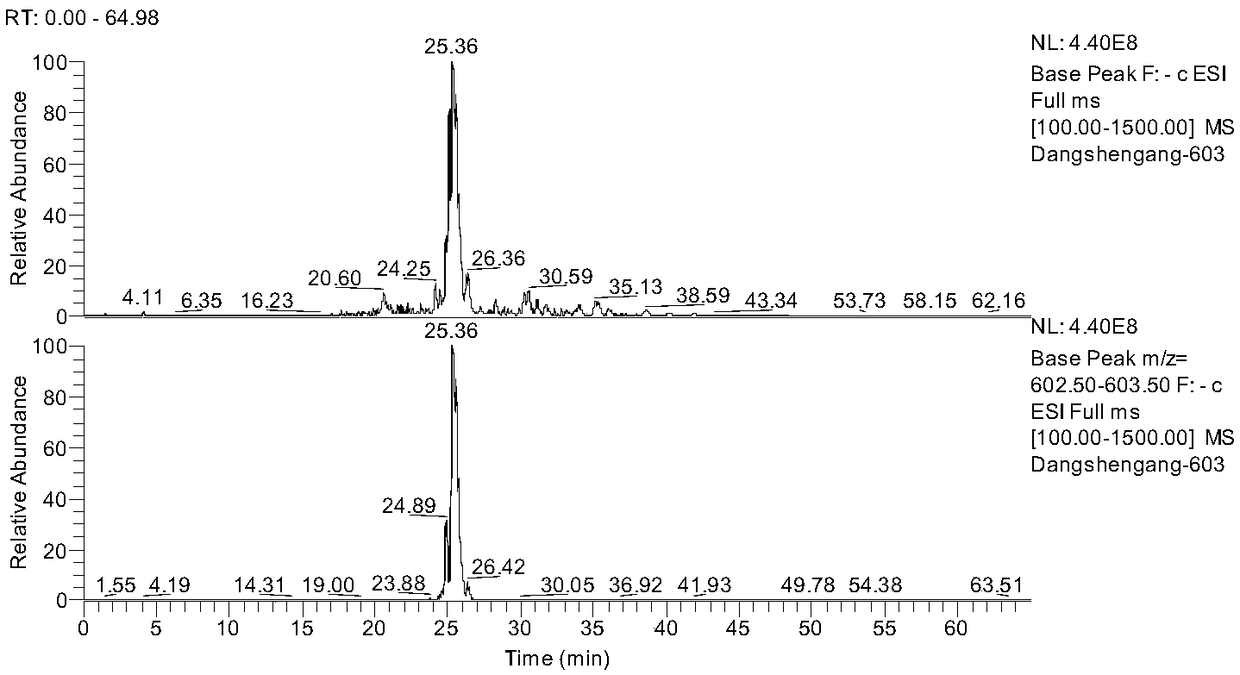
[0031] 8 g of the commercially available Codonopsis glucoside extract (Dausifu, Nanjing) extract was dissolved in 40 mL of pure water by ultrasonic, and the Swiss Buchi pump control system (Pump Manager C-615, PumpModule C-601) were separated and prepared, eluted with pure water, 20%, 40%, 60%, 80%, and 100% ethanol respectively for 3 column volumes, collected and concentrated respectively. The 40% eluate was then eluted with 20%, 22%, 24%, 26%, 28%, 30%, and 100% ethanol for 3 column volumes, and the concentrated solution was collected and concentrated respectively. According to the liquid chromatogram (see attached figure 1 ), the compound Lobetyolinin is mainly concentrated in 22%, 24%, 26% elution part, so 22%, 24%, 26% elution part is then equipped with a semi-preparative column (ZOBARX SB-C18, 21.2×250mm, Agilent) purification, water (A)-acetonitrile (B) is ...
Embodiment 2
[0036] Experiments related to the antiarrhythmic activity of the compounds tangshenoside and lobeyolinin in Example 2:
[0037]2.1. Drugs, reagents, animals and instruments:
[0038] Lobeyolinin, the compound isolated above; Cmlc2-GFP transgenic zebrafish; N-Phenylthiourea (PTU) and Tricaine; Terfenadine; Leica 3000B fluorescence inverted microscope; IX-100F zebrafish ECG recording system.
[0039] 2.2. Zebrafish Arrhythmia Model Establishment
[0040] Cmlc2-GFP transgenic zebrafish is a transgenic line with heart-specific marker green fluorescent protein. Several pairs of heterozygous Cmlc2-GFP transgenic zebrafish females and males were taken, and the embryos were harvested after mating and cultured at a constant temperature of 28°C. After 24 hours of development, the embryos with fluorescence were selected under a fluorescence microscope, and the membranes were ruptured and grouped. Each group had 8-10 embryos, added PTU to inhibit the formation of stains, and cultured at...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com