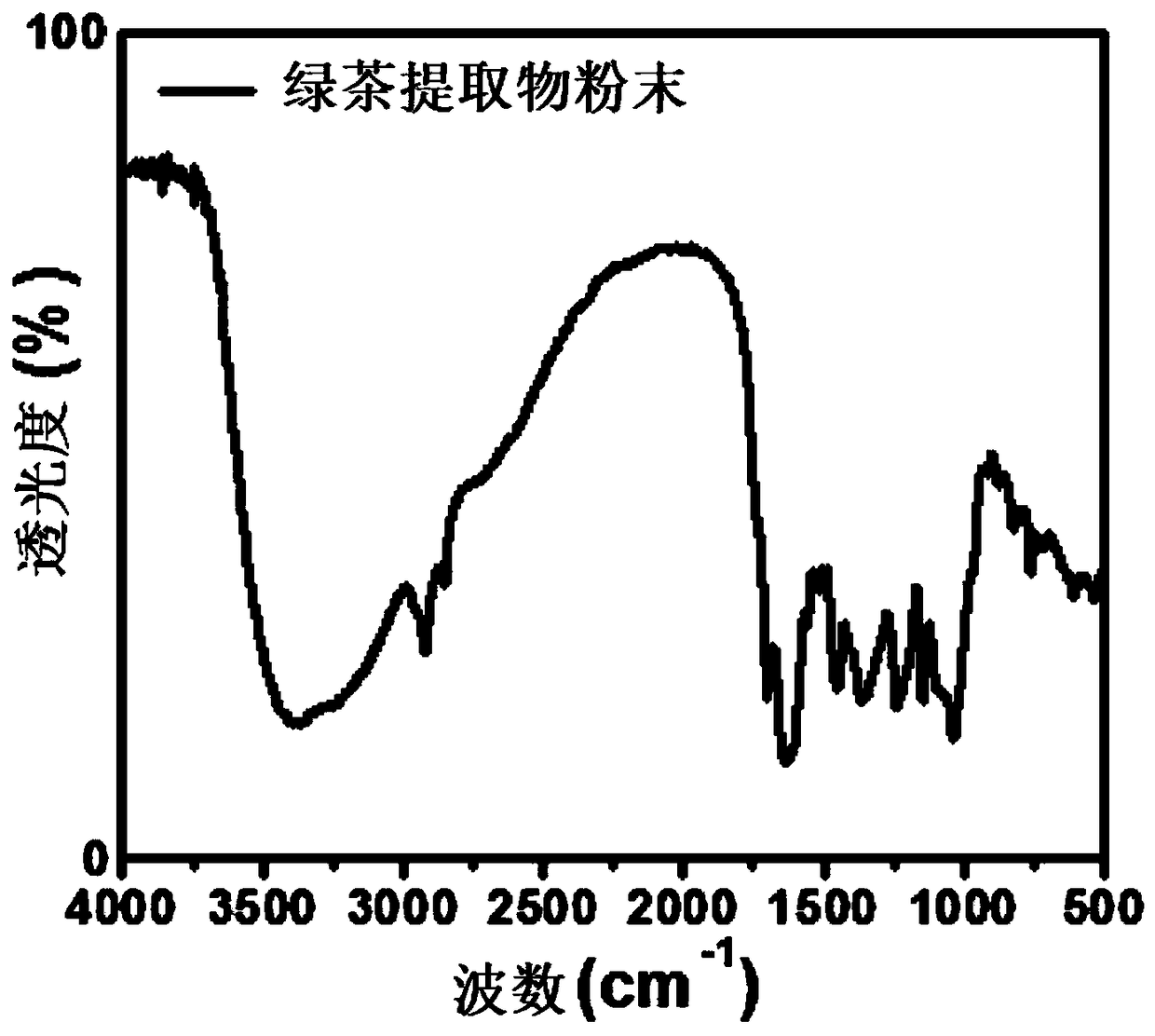
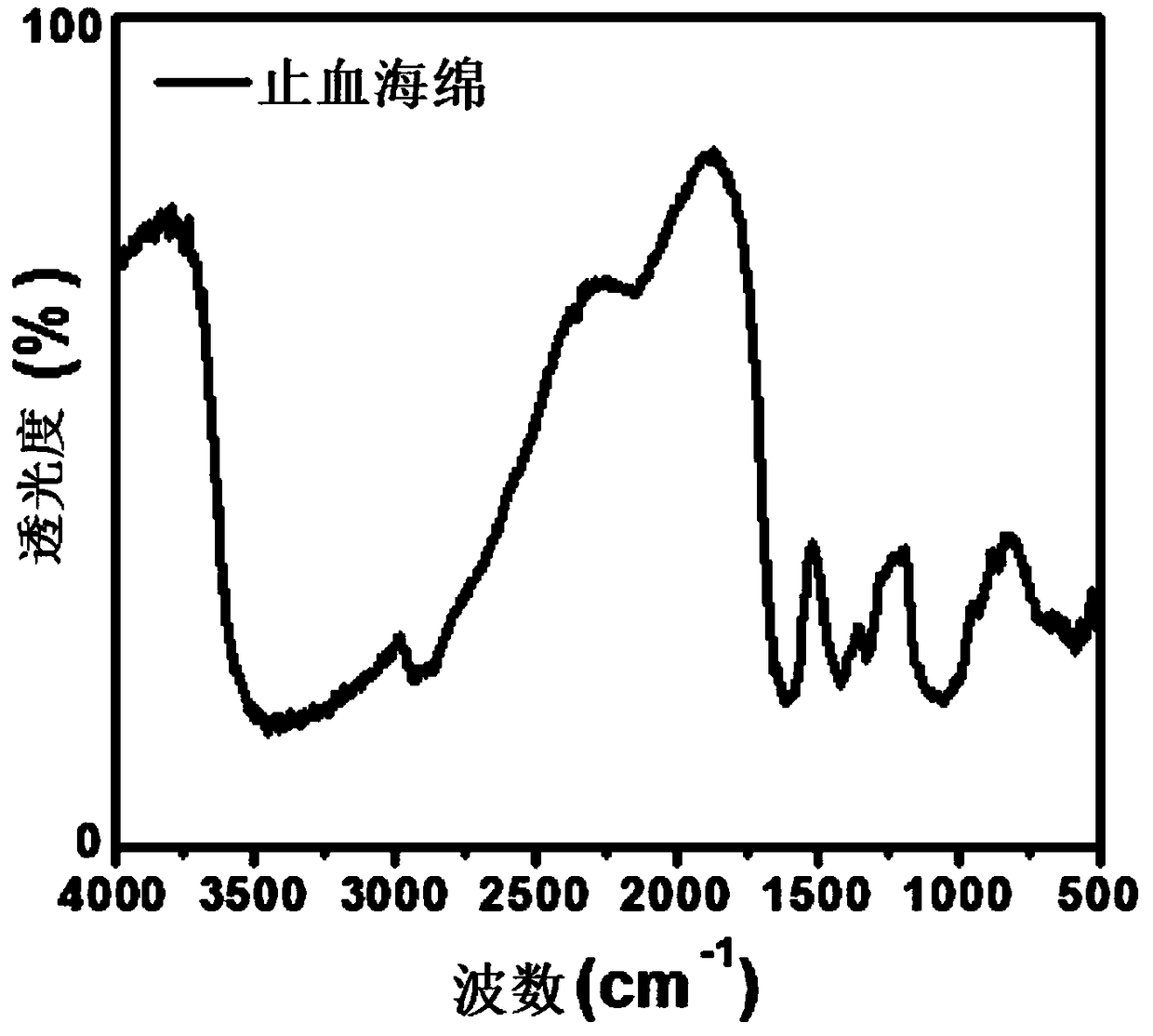
Hemostatic sponge and preparation process thereof
A hemostatic sponge and a preparation process technology, applied in the field of medical supplies, can solve the problems of aldehyde cross-linking agent being easy to produce toxicity, lack of solubility, limited antibacterial performance, etc., and achieve strong infection prevention ability, low production cost and low price. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0019] The embodiment of the present invention relates to a preparation process of a hemostatic sponge, comprising the following steps:
[0020] S 1 , add carboxymethyl chitosan to deionized water, dissolve completely, obtain carboxymethyl chitosan polymer solution; add chitosan to carboxymethyl chitosan polymer solution, mix evenly, obtain polymer mixture Solution; in parts by weight, chitosan in the polymer mixed solution is 0.1-0.5 parts, carboxymethyl chitosan is 1-5 parts, and deionized water is 10-100 parts;
[0021] S 2 , add 0.1-0.5 parts by weight of green tea extract powder to the polymer mixed solution, mix well, then add glycerin for foaming, place the foamed solution in a 10×10cm plastic petri dish, and then add calcium chloride solution Promote the cross-linking of chitosan and carboxymethyl chitosan to obtain a cross-linked polymer solution;
[0022] S 3 , freeze-dried to obtain a hemostatic sponge, and finally cut into shape.
[0023] In parts by weight, p...
Embodiment 1
[0032] This embodiment relates to a preparation process of a hemostatic sponge, comprising the following steps:
[0033] S 1 , add 5g carboxymethyl chitosan to 100g deionized water, dissolve completely, obtain carboxymethyl chitosan polymer solution; Add 0.5g chitosan in carboxymethyl chitosan polymer solution, mix well, Obtain polymer mixed solution;
[0034] S 2 , add 0.2g of green tea extract powder to the polymer mixed solution, mix evenly, then add 1ml of glycerin to foam for 30min, add 5ml of calcium chloride solution with a solution concentration of 1% after foaming to promote chitosan and carboxymethyl shell Glycans are cross-linked to obtain a cross-linked polymer solution;
[0035] S 3 , put the cross-linked polymer solution at -20~-80°C for 24 hours, then take it out and immediately freeze-dry it for 24 hours to obtain a hemostatic sponge, which is finally cut into shape and sterilized by UV, and then stored in a dry box at 37°C in spare.
[0036] The green te...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com