Application of icarisid I type compound for preparing IDO inhibitor
A technology of icariin and compound, which is applied in the field of preparing IDO inhibitor and icariin class I compound
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
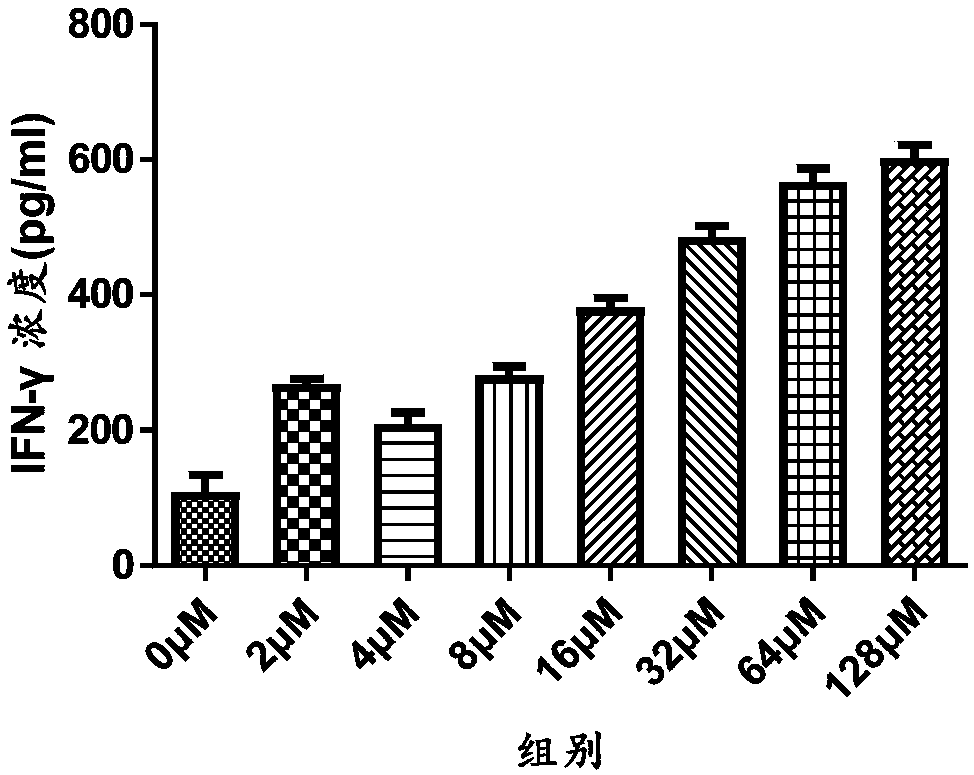
[0027] Example 1: Effect of Icariside I on the Change of IFN-γ Content in T Cell Culture Supernatant
[0028] Prepare preparations for culturing cells according to conventional methods, recover and cultivate cells for experiments using conventional methods.
[0029] Collect freshly isolated or purchased CD3 + T cells were counted with a counting plate under an inverted microscope, seeded in a 96-well plate with 25,000 to 50,000 cells per well, and then added different concentrations of icariside Ⅰ prepared with medium and placed in a 5% CO 2 After cultured in the cell culture box for 48 hours, the supernatant of the cells was collected and stored in a freezer at -80°C, or the IFN-γ content secreted by T cells at different concentrations was detected directly with the IFN-γELISA kit, and calculated by GraphPad Prism 5 And plotted to get the effect of icariside Ⅰ on CD 3 at different concentrations + The effect of changes in the content of IFN-γ secreted by T cells, the result...
Embodiment 2
[0031] Example 2: Experimental procedures for the regulation of apoptotic protein expression by Western blot of icariin I
[0032] (1) Sample treatment: cells were seeded in a 96-well plate at a concentration of 5000 cells / well, 5 times the number of T cells were added to each well, and then different concentrations of icariside I diluted with medium were added to act Cells were collected after 48 hours and washed twice with PBS;
[0033] (2) Extraction of total protein: the liver cancer cells added to the plate were collected by centrifugation and washed twice with phosphate buffered saline. Add an appropriate amount of lysate to the cells, let stand on ice for half an hour, centrifuge at 12,000 rpm for 10 minutes in a refrigerated centrifuge, and transfer the supernatant to a new centrifuge tube. The BCA method is used to detect the protein concentration in the sample, add denaturing solution in a 95°C water bath for 8-10 minutes to denature the protein, and store it in a ref...
Embodiment 3
[0040] Example 3: Regulation of Icariside I on the Expression of Apoptotic Proteins
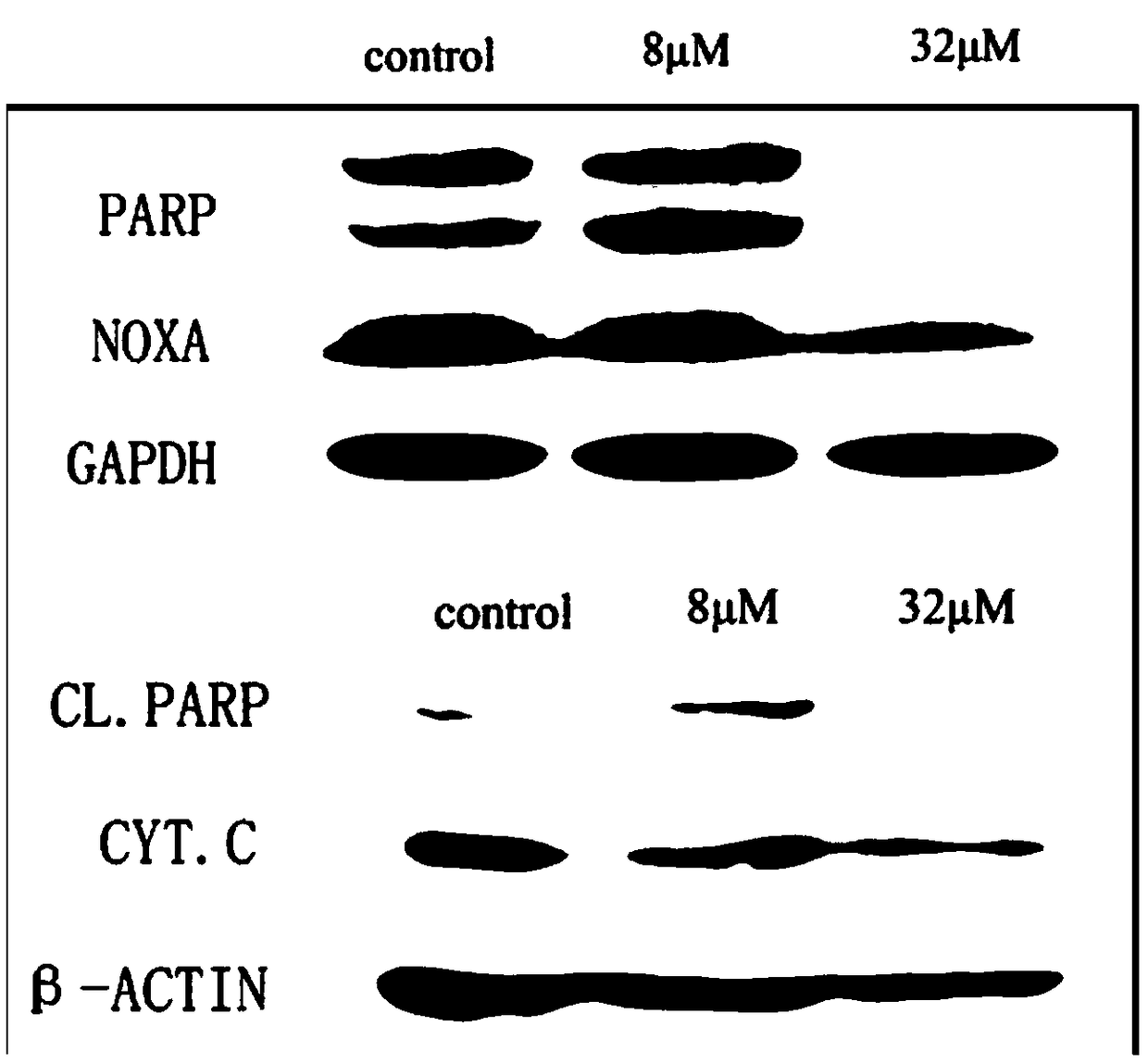
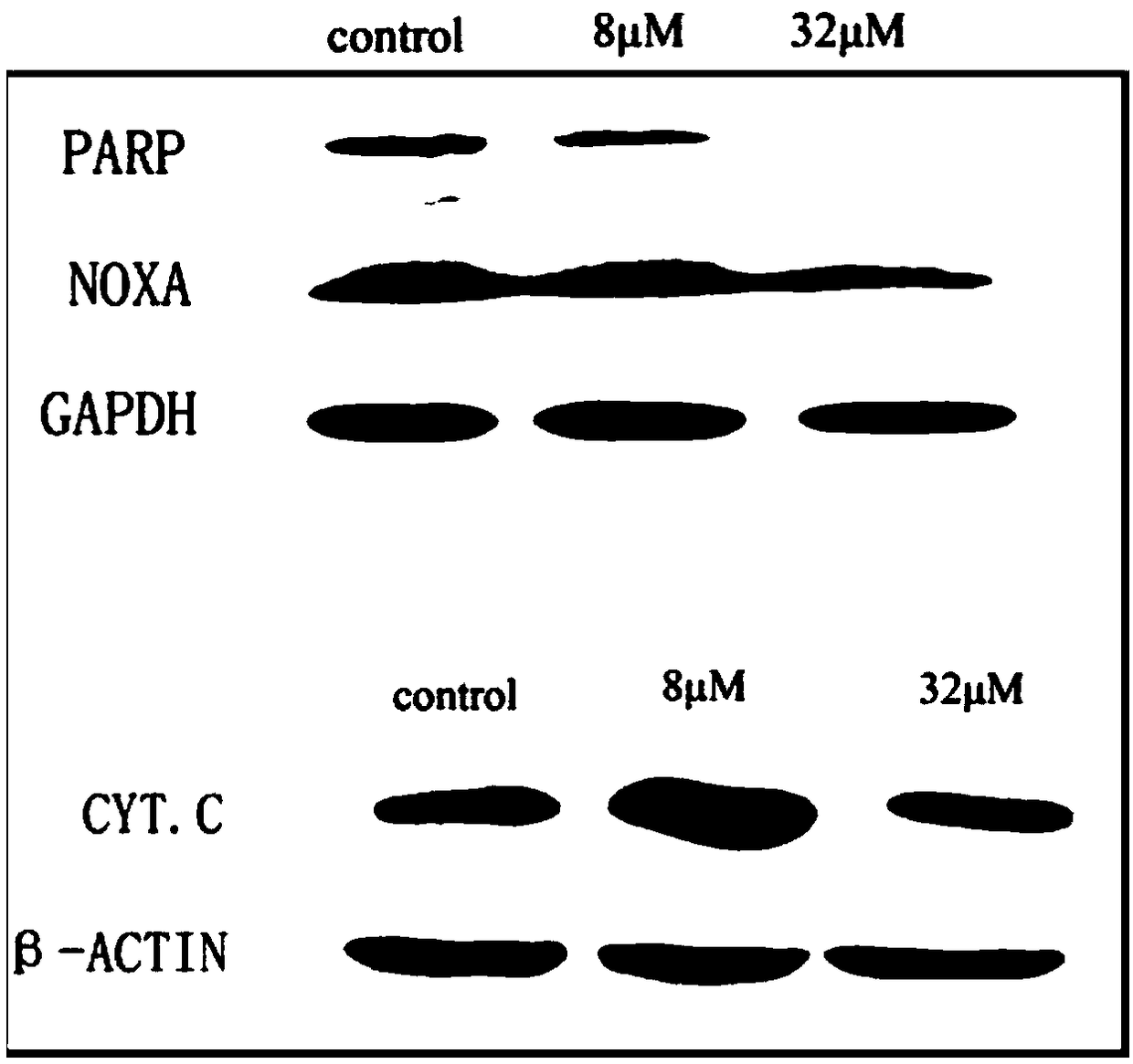
[0041] The Western blot experiment investigated the regulation of icariside Ⅰ on the expression of major apoptotic proteins in the IFN-γ-mediated apoptosis pathway in liver cancer cells at high concentrations of 8 μM and 32 μM. The results are as follows: figure 2 with image 3 shown.
[0042] figure 2 with image 3 It is the result of western blot experiment on liver cancer cells treated with 8 μM and 32 μM icariside Ⅰ for 48 hours in the presence of T cells. Here, the proteins related to the apoptosis cascade pathway (including PARP / cleaved PARP), as well as the marker proteins of apoptosis Noxa and Cytochrome C were mainly detected. The results showed that the expressions of apoptosis marker proteins Cytochrome C and cleaved PARP in HepG2 and Huh7 cells treated with icariside Ⅰ were significantly increased when the administration concentration was below 8 μM, and the expression of No...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com