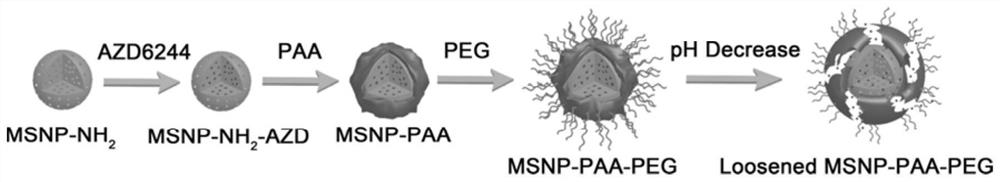
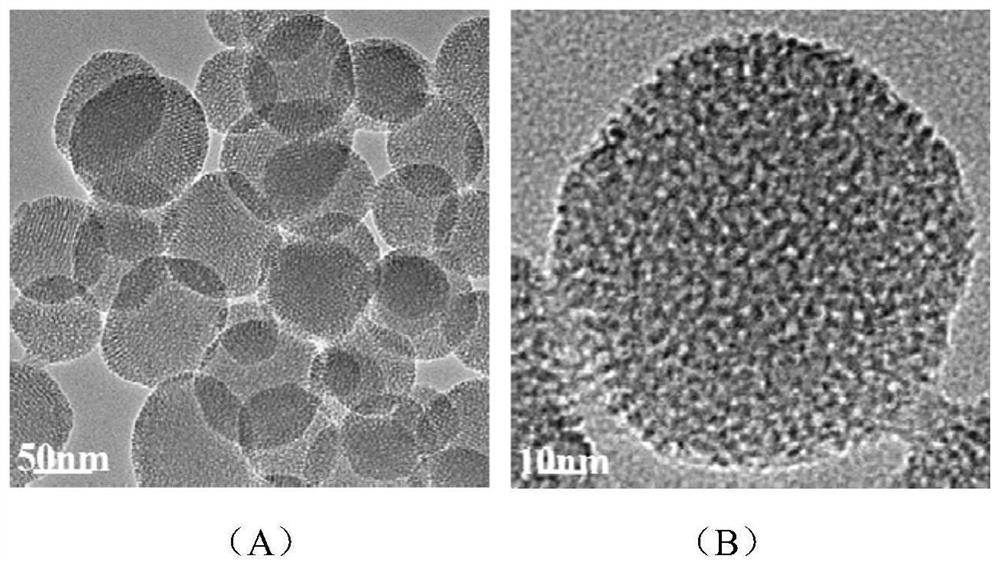
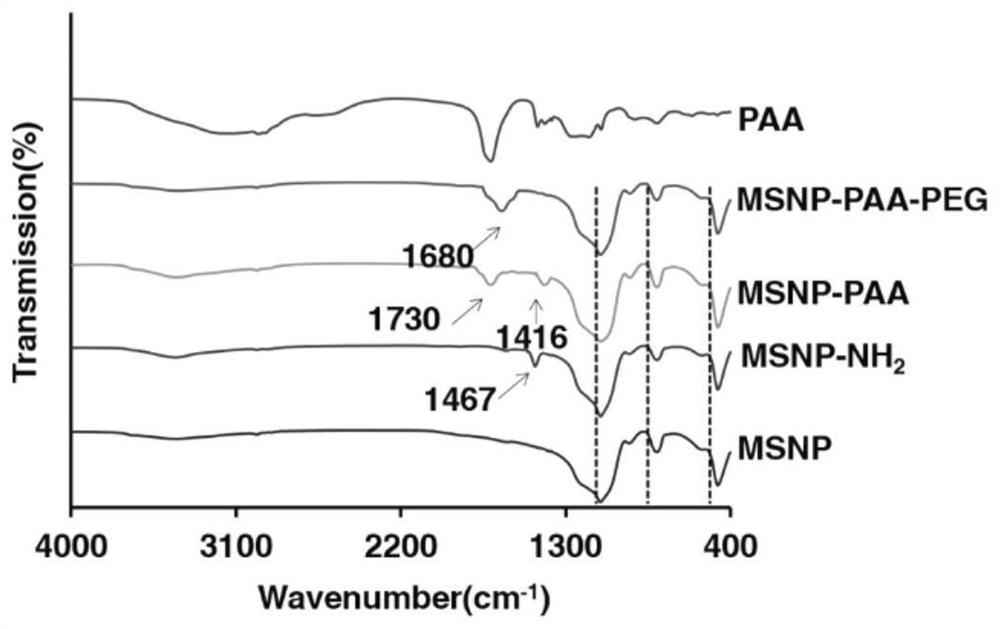
Mesoporous silica nanometer drug-loaded particles targeting tumor tissue and cells and preparation method thereof
A technology of mesoporous silica and nano-drug loading, which is applied in the direction of anti-tumor drugs, pharmaceutical formulations, medical preparations of non-active ingredients, etc., to achieve large specific surface area and pore volume, good biological safety, uniform and controllable media Effect of Hole Size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] In this example, the small-molecule targeted drug MEK inhibitor AZD6244 was used as the drug to be loaded to prepare mesoporous silica nanoparticles drug-loaded particles with dual targeting of tumor tissue and cells. The steps are as follows:
[0065] (1) Preparation of mesoporous silica nanoparticles (MSNP)
[0066] Dissolve 100 mg of cetyltrimethylammonium bromide (CTAB) in deionized water to form a CTAB aqueous solution with a concentration of 2.1 mg / mL, adjust the pH value of the CTAB aqueous solution to 11.0 with 2 mol / L NaOH solution, and stir in a water bath Heat to 80°C, and after the temperature stabilizes, add tetraethyl orthosilicate dropwise into the CTAB aqueous solution at a rate of 180 μL / min according to the volume ratio of tetraethyl orthosilicate (TEOS) and CTAB aqueous solution at a ratio of 1:96 , stirred and reacted at 80°C for 3h to obtain a white precipitate, then centrifuged at a speed of 10000rpm for 15min, collected the precipitate, and disperse...
Embodiment 2
[0088] In this example, the drug release rate of MSNP-PAA-PEG prepared in Example 1 in different pH environments was tested.
[0089] The MSNP-PAA-PEG prepared in Example 1 was placed in PBS buffers with different pH conditions (pH=7.4, pH=6.8, pH=5.5, pH=4.5), samples were centrifuged at intervals, and the supernatant was taken. The drug release rate was tested by HPLC. The test conditions of HPLC are: mobile phase, methanol:water=8:2 (v:v), flow rate 1mL / min, detection wavelength 257.8nm, injection volume 20 μ L.
[0090] The release curves of MSNP-PAA-PEG under different pH conditions are as follows: Figure 8 shown by Figure 8 It can be seen that the release rate of drug AZD6244 is 9.9%, 20.1%, 49.82% and 71.02% under the conditions of pH value 7.4, 6.5, 5.5 and 4.5, and the drug release rate increases significantly with the decrease of pH value. Since the pH value of the normal pH environment in the body is about 7.4, it is not enough to protonate the PAA in the PAA f...
Embodiment 3
[0092] In this example, the in vitro cell uptake level and in vivo biodistribution of FITC-loaded mesoporous silica nanoparticles MSNP-PAA-PEG-FITC prepared in Comparative Example 1 were tested.
[0093] Melanoma cells SMM101 and SMM103, lymphocytes EL4T, Jurkat and mouse spleen lymphocytes were treated with MSNP-PAA-PEG-FITC at a final concentration of 50 μg / mL and 25 μg / mL for 24 h, 48 h and 72 h, and then analyzed by flow cytometry The uptake of MSNP-PAA-PEG-FITC in tumor cells and lymphocytes was detected by cytometer and confocal microscope, and the results were as follows: Figures 9 to 11 as shown, Figure 9 is the endocytosis ratio of MSNP-PAA-PEG-FITC detected by flow cytometry in tumor cells. Figure 10 is the endocytosis ratio of MSNP-PAA-PEG-FITC detected by flow cytometry in lymphocytes. Figure 11 It is the result of quantitative analysis of MSNP-PAA-PEG-FITC in vitro tumor and lymphocyte uptake levels. Depend on Figures 9 to 11It can be seen that the uptake...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com