Method for preparing p-hydroxy phenyl ethyl ketone
A technology for p-hydroxyacetophenone and phenyl acetate, which is applied in the field of preparing p-hydroxyacetophenone, can solve the problems of difficult reaction control, long reaction time, troublesome post-processing, etc., and achieves easy automatic control, stable reaction, and product yield. High rate and purity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
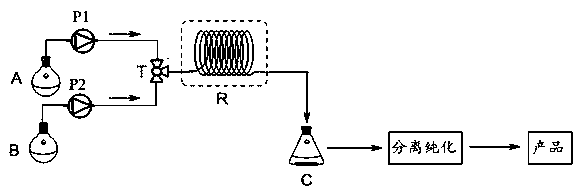
[0024] Add phenyl acetate (13.6kg, 100mol) into the first storage tank A, dissolve it with chloroform until it is clear, and accurately adjust the volume to 25 L to make a solution with a concentration of phenyl acetate of 4.0 mol / L, stir well and seal it for later use. Add aluminum trichloride (20 kg, 150 mol) into the second storage tank B, dissolve it with chloroform until it is clear, and accurately adjust the volume to 200 L to make a solution with a concentration of aluminum trichloride of 0.75 mol / L and seal it for use .
[0025] The materials in the first storage tank A and the second storage tank B are transported through the first metering pump P1 and the second metering pump P2 respectively, and enter the mixer T for mixing, and the mixed raw material liquid continuously enters the tubular reactor for Fries rearrangement Reaction (the inner diameter of the pipeline is 0.31 cm, the length of the pipeline is 50m, and the molar flow ratio of phenyl acetate: aluminum t...
Embodiment 2
[0027] Add phenyl acetate (13.6kg, 100mol) into the first storage tank A, dissolve it with nitromethane until it is clear, and accurately adjust the volume to 25L to make a solution with a phenyl acetate concentration of 4.0 mol / L, stir well and seal it stand-by. Add aluminum trichloride (20 kg, 150 mol) into the second storage tank B, dissolve it with nitromethane until it is clear, and accurately adjust the volume to 200 L to make a solution with a concentration of aluminum trichloride of 0.75 mol / L and seal it stand-by.
[0028] The materials in the first storage tank A and the second storage tank B are transported through the first metering pump P1 and the second metering pump P2 respectively, and enter the mixer T for mixing, and the mixed raw material liquid continuously enters the tubular reactor for Fries rearrangement Reaction (the inner diameter of the pipeline is 0.31 cm, the length of the pipeline is 50m, and the molar flow ratio of phenyl acetate: aluminum trichl...
Embodiment 3
[0031] Add phenyl acetate (13.6kg, 100mol) into the first storage tank A, dissolve it with chlorobenzene until it is clear, and accurately adjust the volume to 25 L to make a solution with a concentration of phenyl acetate of 4.0 mol / L, stir well and seal it stand-by. Add aluminum trichloride (20 kg, 150 mol) into the second storage tank B, dissolve it with chlorobenzene until it is clear, and accurately adjust the volume to 200 L to make a solution with a concentration of aluminum trichloride of 0.75 mol / L. use.
[0032] The materials in the first storage tank A and the second storage tank B are transported through the first metering pump P1 and the second metering pump P2 respectively, and enter the mixer T for mixing, and the mixed raw material liquid continuously enters the tubular reactor for Fries rearrangement Reaction (the inner diameter of the pipeline is 0.31 cm, the length of the pipeline is 50m, and the molar flow ratio of phenyl acetate: aluminum trichloride is c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com