Preparation method of lilial intermediate p-tert-butyl-alpha-methylcinnamaldehyde
A technology of methyl cinnamaldehyde and p-tert-butyl, which is applied in the field of fragrance chemistry and fine chemicals, can solve the problems of difficult operation, easy blockage of pipelines, and high energy consumption of separation, so as to avoid rectification and recovery of solvents and improve product quality. Quality and the effect of reducing the amount of three wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
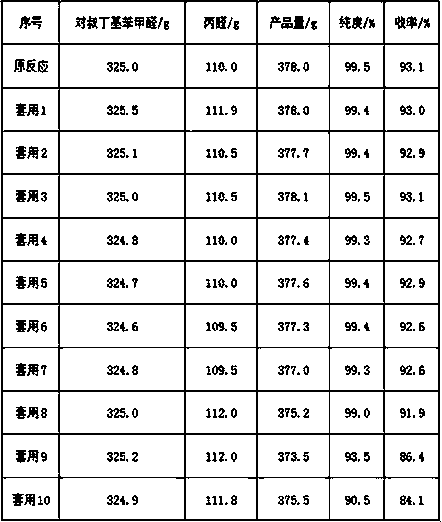
Image
Examples
Embodiment 1
[0044] Embodiment 1 A kind of preparation method of lyral intermediate p-tert-butyl-alpha-methylcinnamaldehyde
[0045] Include the following steps:
[0046] (1) Feeding
[0047] In a reactor with a stirring device, a reflux device, a thermometer and a dropping funnel, add 400 g of n-butanol and 5.0 g of potassium hydroxide, stir, and until the dissolution of the potassium hydroxide is complete, add 325 g of p-tert-butylbenzaldehyde.
[0048] (2) Add propionaldehyde dropwise
[0049] At 24°C, the dropwise addition of propionaldehyde (110 g) was started, and the dropwise addition of propionaldehyde was completed after 3 hours.
[0050] (3) Insulation reaction
[0051] Insulate and react at 40°C, and take samples every 0.5h for detection. After the content of p-tert-butylbenzaldehyde is found to be basically unchanged, stop heating and the reaction ends.
[0052] (4) crystallization
[0053] The temperature was lowered slowly, crystals were precipitated, and the temperature w...
Embodiment 2
[0058] Embodiment 2 A kind of preparation method of lyral intermediate p-tert-butyl-alpha-methylcinnamaldehyde
[0059] Include the following steps:
[0060] (1) Feeding
[0061] In a reactor with a stirring device, a reflux device, a thermometer and a dropping funnel, add 400.0 g of isopropanol and 1.6 g of potassium hydroxide and stir. After the potassium hydroxide is completely dissolved, add 325.0 g of p-tert-butylbenzaldehyde .
[0062] (2) Add propionaldehyde dropwise
[0063] At 15°C, 95.0 g of propionaldehyde was added dropwise, and the dropwise addition of propionaldehyde was completed after 2 hours.
[0064] (3) Insulation reaction
[0065] Insulate and react at 45°C, and take samples every 0.5h for detection. After the content of p-tert-butylbenzaldehyde remains basically unchanged, stop heating and complete the reaction.
[0066] (4) crystallization
[0067] The temperature was lowered slowly, crystals were precipitated, and the temperature was lowered to 20°...
Embodiment 3
[0070] Embodiment 3 A kind of preparation method of lylial intermediate p-tert-butyl-alpha-methylcinnamaldehyde
[0071] Include the following steps:
[0072] (1) Feeding
[0073] In a reactor with a stirring device, a reflux device, a thermometer and a dropping funnel, add 400.0 g of isopropanol and 6.5 g of lithium hydroxide, stir, and after the dissolution of lithium hydroxide is complete, add 325.0 g of p-tert-butylbenzaldehyde.
[0074] (2) Add propionaldehyde dropwise
[0075] At 20°C, 105.0 g of propionaldehyde was added dropwise, and the dropwise addition was completed after 4 hours.
[0076] (3) Insulation reaction
[0077] Insulate and react at 45°C, and take samples every 0.5h for detection. After the content of p-tert-butylbenzaldehyde remains basically unchanged, stop heating and complete the reaction.
[0078] (4) crystallization
[0079] The temperature was lowered slowly, crystals were precipitated, and the temperature was lowered to 20°C and kept for 1 ho...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com