Halogenated lead-caesium perovskite fluorescent material and preparation method thereof
A fluorescent material, perovskite technology, applied in the field of fluorescent materials, can solve the problems of easy decomposition, fluorescence quenching, loss of optical properties, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0031] The present invention provides the preparation method of the lead-halide cesium perovskite fluorescent material described in the above technical scheme, comprising the following steps:
[0032] Under the condition of stirring, the protonated carbon nitride dispersion liquid is added dropwise into the lead halide cesium quantum dot dispersion liquid, and the protonated carbon nitride and the halide lead cesium quantum dots self-assemble to obtain the lead halide cesium perovskite fluorescent material.
[0033] In the present invention, the solvent in the protonated carbon nitride dispersion is preferably N,N-dimethylformamide (DMF), and the concentration of the protonated carbon nitride dispersion in the protonated carbon nitride dispersion is preferably 8-12 mg / mL, more preferably 10 mg / mL. The present invention has no special limitation on the preparation method of the protonated carbon nitride dispersion liquid, and the preparation method well known to those skilled i...
Embodiment 1
[0051] Preparation of protonated carbon nitride dispersion includes the following steps:
[0052] In nitrogen protection, urea is heated from room temperature to 550 °C at a rate of 5 °C / min, and heat-treated for 2 hours at an insulated temperature; after completing the heat treatment, it is naturally cooled to room temperature and ground to obtain carbon nitride (g-C 3 N 4 );
[0053] 10 mg of the carbon nitride was mixed with 2 mL of HBr solution (mass concentration: 48%), and ultrasonically performed protonation treatment for 10 h. After the protonation treatment was completed, the obtained system was centrifuged at a speed of 6500 r / min for 10 min. The obtained supernatant was evaporated to dryness in an oil bath at 80°C, and then the obtained residue was vacuum-dried at 50°C for 12 hours, and the obtained light yellow powder was protonated carbon nitride (PCN);
[0054] Weigh 10 mg of the protonated carbon nitride and 1 mL of N,N-dimethylformamide and ultrasonically mix...
Embodiment 2
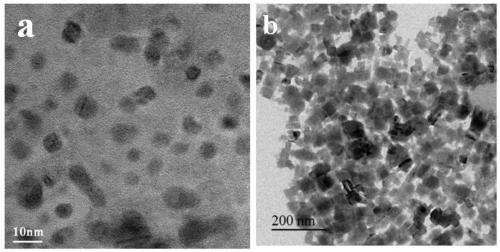
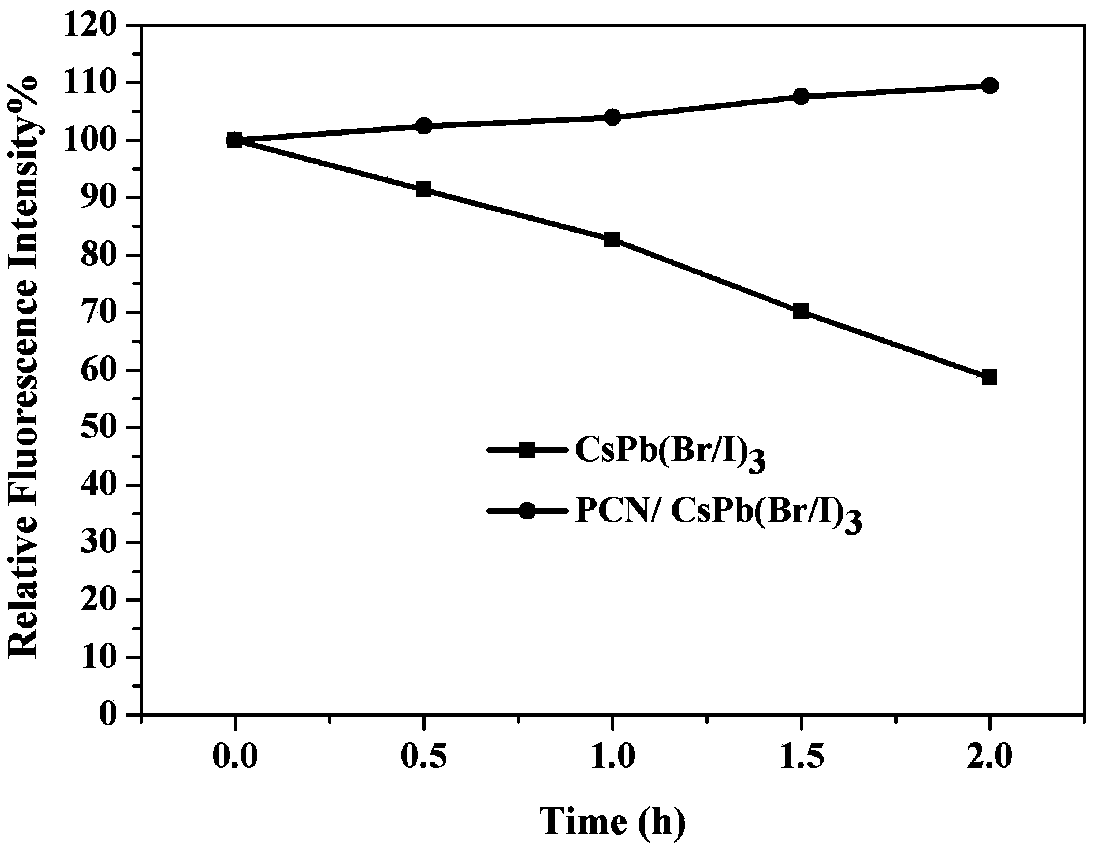
[0062] Prepare the lead-halide cesium perovskite fluorescent material according to the method of Example 1, the difference is that when preparing the lead-halide cesium quantum dot dispersion, CsPbBr 3 The consumption of stock solution is 0.8mL, ZnI 2 The amount of N,N-dimethylformamide solution is 0.04mL; then use the obtained 20.84mL lead halide cesium quantum dot dispersion (CsPb(Br / I) 3 dispersion, the CsPb(Br / I) 3 The molar ratio of Br and I is 8:2, which can be specifically expressed as CsPb(Br0.8 / I 0.2 ) 3 ) and 91.75 μL protonated carbon nitride dispersion liquid to prepare halide-lead-cesium perovskite fluorescent material (PCN / CsPb(Br / I) 3 , specifically expressed as PCN / CsPb(Br 0.8 / I 0.2 ) 3 ).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Granularity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com