Method for removing calcium ions from manganese sulfate solution
A manganese sulfate solution and calcium ion technology, applied in the field of hydrometallurgy, can solve problems such as wasting manganese resources, and achieve the effects of improving quality, saving energy and auxiliary materials, and improving metal yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
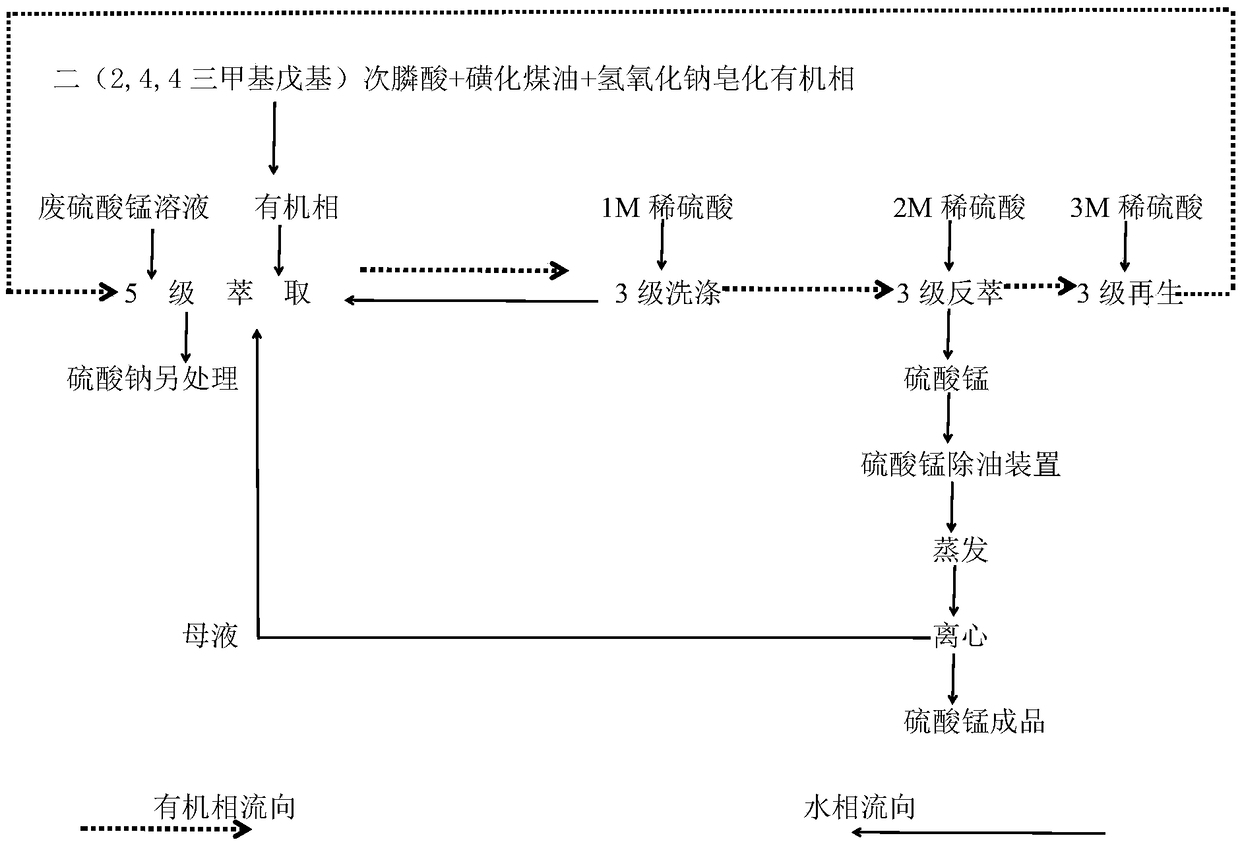
[0031] In this example, before extraction: the pH value of the aqueous phase is 1.0 to 2.0, the calcium ion content in the aqueous phase is 0.4 g / L, the manganese ion content is 20 g / L, and the bis(2,4,4 trimethyl The concentration of pentyl) phosphinic acid is 15%, and the saponification rate is 40%; in the extraction reaction process: the extraction ratio is 4.5:1, the washing ratio is 1:8, and the stripping ratio is 1:15, the extraction, In the three stages of washing and stripping, the reaction temperature is 35°C, the reaction time is 3 minutes, and the rotation speed of the stirring paddle is controlled at 300 rpm; after multi-stage countercurrent extraction, washing and stripping, the manganese sulfate solution: pH The value is 2.5, the manganese ion content is 70g / L, and the calcium ion content is 0.01g / L.
Embodiment 2
[0033] In this example, before extraction: the pH value of the aqueous phase is 1.0 to 2.0, the calcium ion content in the aqueous phase is 0.5 g / L, the manganese ion content is 25 g / L, and the bis(2,4,4 trimethyl The concentration of pentyl) phosphinic acid is 15%, and the saponification rate is 50%; in the extraction reaction process: the extraction ratio is 5:1, the washing ratio is 1:6, and the stripping ratio is 1:18, the extraction, In the three stages of washing and stripping, the reaction temperature is 35°C, and the reaction time is 3 minutes; the rotation speed of the stirring paddle is controlled at 300 rpm; after multi-stage countercurrent extraction, washing and stripping, multi-stage countercurrent extraction , After washing and stripping, manganese sulfate solution: pH value is 2.2, manganese ion content is 90g / L, calcium ion content is 0.012g / L.
Embodiment 3
[0035] In this example, before extraction: the pH value of the aqueous phase is 1.0 to 2.0, the calcium ion content in the aqueous phase is 0.3 g / L, the manganese ion content is 30 g / L, and the bis(2,4,4 trimethyl The concentration of pentyl) phosphinic acid is 15%, and the saponification rate is 50%; in the extraction reaction process: the extraction ratio is 6:1, the washing ratio is 1:8, and the stripping ratio is 1:20, the extraction, In the three stages of washing and stripping, the reaction temperature is 35°C, the reaction time is 3 minutes, and the rotation speed of the stirring paddle is controlled at 300 rpm; after multi-stage countercurrent extraction, washing and stripping, multi-stage countercurrent extraction , After washing and stripping, manganese sulfate solution: pH value is 2.8, manganese ion content is 98g / L, calcium ion content is 0.008g / L.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com