Child ibuprofen taste masking dry suspension and preparation method thereof
A dry suspension and children's technology, which is applied in the field of ibuprofen taste-masked dry suspension for children and its preparation, can solve the problem that it is difficult to meet the clinical treatment needs of infants and children with acute fever, and the individualized difference in antipyretic time is large. , children's poor medication compliance and other problems, to achieve the effect of improving medication compliance, improving physical stability, and improving pungency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
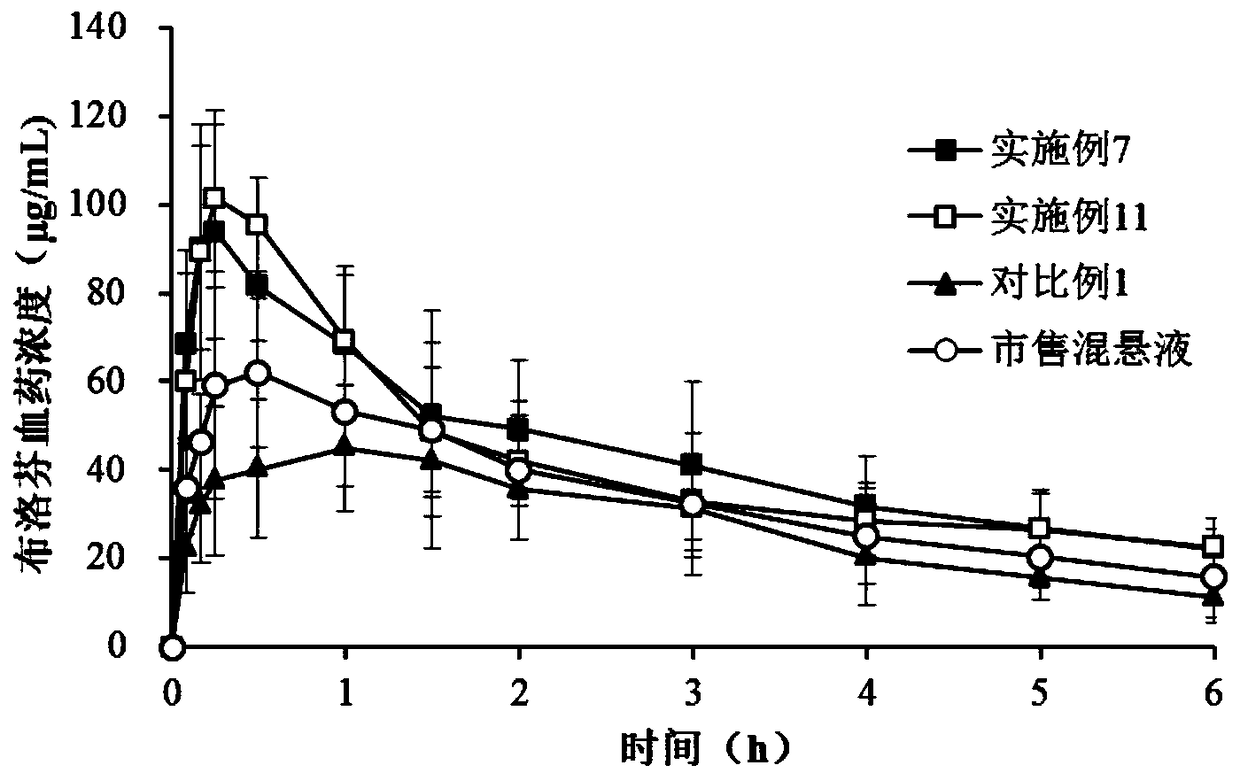
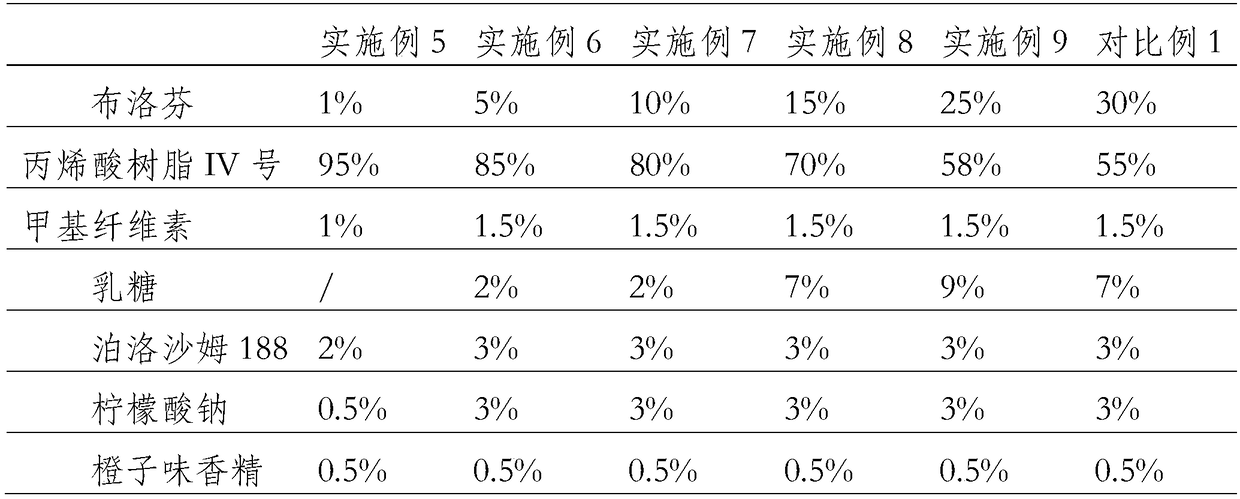
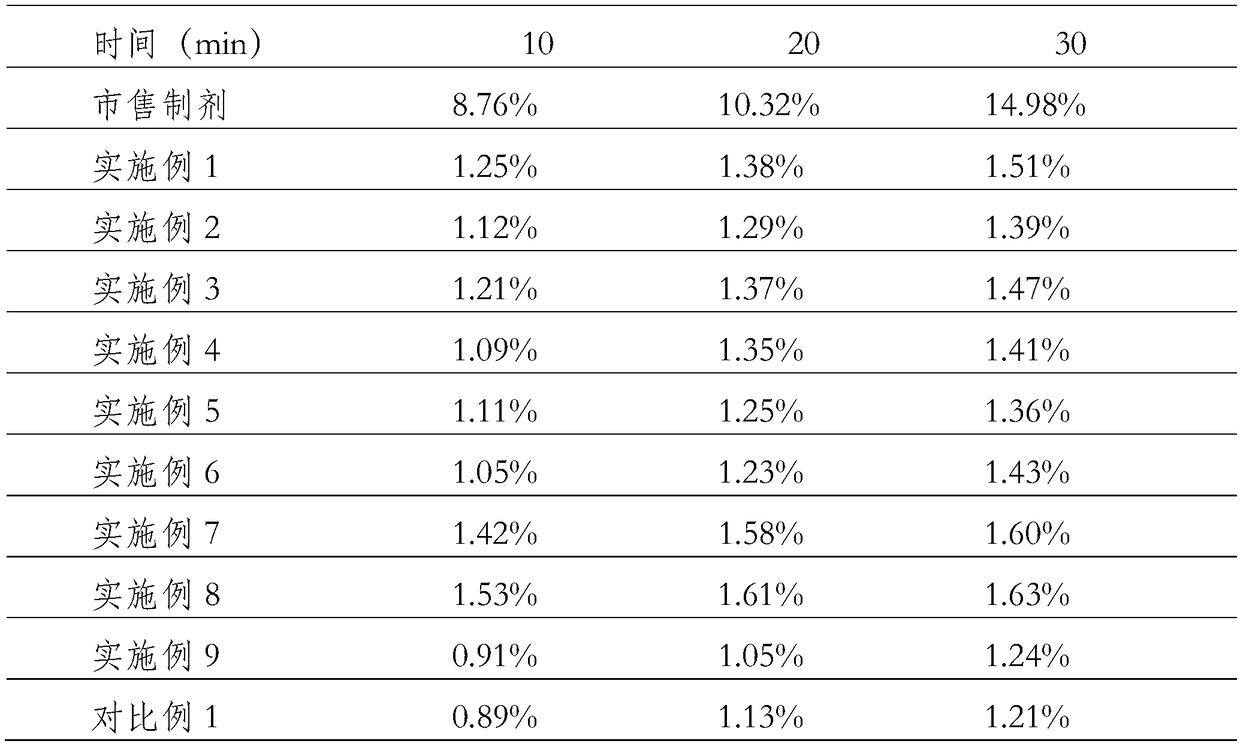
Image
Examples
Embodiment 1
[0029] The present embodiment prepares 100g ibuprofen taste-masking dry suspension preparation for children, which is as follows in terms of the mass percentage of each material component:
[0030]Ibuprofen: 5%; Acrylic No. IV: 85%; Gum Arabic: 1%; Sucrose: 2%;
[0031] Poloxamer 188: 3%; Sodium Citrate: 3%; Stevioside: 0.5%
[0032] Preparation method: (1) Grind the prescribed amount of ibuprofen, acrylic resin No. IV, gum arabic, lactose, poloxamer 188, sodium citrate, and orange flavor, and pass through a 40-mesh sieve.
[0033] (2) Mix the ibuprofen and acrylic resin IV in the step (1) uniformly according to the above mass ratio to obtain a uniform mixture.
[0034] (3) The temperature of the hot-melt extruder is set to 70° C., and the rotation speed is 10 rpm. When the temperature and rotating speed rise to the set value, put the mixture prepared in step (2) into the material hopper of hot-melt extrusion, collect the extrudate at the material outlet of the hot-melt extr...
Embodiment 2
[0038] The present embodiment prepares 100g ibuprofen taste-masking dry suspension for children, which is as follows in terms of the mass percentage of each material component:
[0039] Ibuprofen: 5%; Acrylic No. IV: 85%; Gum Arabic: 1%; Lactose: 2%;
[0040] Poloxamer 188: 3%; Sodium Citrate: 3%; Orange Flavor: 1.0%
[0041] Preparation method: (1) Grind the prescribed amount of ibuprofen, acrylic resin No. IV, gum arabic, lactose, poloxamer 188, sodium citrate, and orange flavor, and pass through a 60-mesh sieve.
[0042] (2) Dissolve ibuprofen and acrylic resin No. IV in ethanol to obtain a uniform solution.
[0043] (3) Spray-dry the solution obtained in step (2), and the operating parameters are as follows: inlet air temperature 40°C, spray pressure 100KPa, outlet air temperature 40°C, after spray drying, maintain for 10 minutes for secondary drying. The ibuprofen-polymer carrier solid dispersion is obtained.
[0044] (4) Mix the dry granules obtained in step (3) with ...
Embodiment 3
[0046] The present embodiment prepares 100g ibuprofen taste-masking dry suspension for children, which is as follows in terms of the mass percentage of each material component:
[0047] Ibuprofen: 5%; Acrylic resin No. IV: 85%; Gum Arabic: 1%; Dextrin: 2%;
[0048] Poloxamer 188: 3%; Sodium Citrate: 3%; Orange Flavor: 0.5%
[0049] Preparation method: (1) Grind the prescribed amount of ibuprofen, acrylic resin No. IV, gum arabic, lactose, poloxamer 188, sodium citrate, and orange flavor, and pass through a 100-mesh sieve.
[0050] (2) The ibuprofen in the step (1) and the acrylic resin No. IV are mixed uniformly according to the above mass ratio to form a uniform mixture.
[0051] (3) heating and melting the homogeneous material obtained in step (2) to obtain a molten liquid.
[0052] (4) Spray and freeze the molten liquid obtained in step (3), enter air temperature at -5°C, spray speed at 2mL / min, and spray pressure at 100KPa to obtain the ibuprofen-polymer carrier solid di...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Glass transition temperature | aaaaa | aaaaa |
| Glass transition temperature | aaaaa | aaaaa |
| Glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com