A kind of α-acetyl-γ-butyrolactone sodium salt free acetamidine hydrochloride process
A technology of acetamidine hydrochloride and butyrolactone, which is applied in the direction of organic chemistry and the like, can solve the problems of complex preparation process of α-acetyl-γ-butyrolactone, low income, environmental pollution and the like, so as to reduce the synthesis cost and improve the benefit. , the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
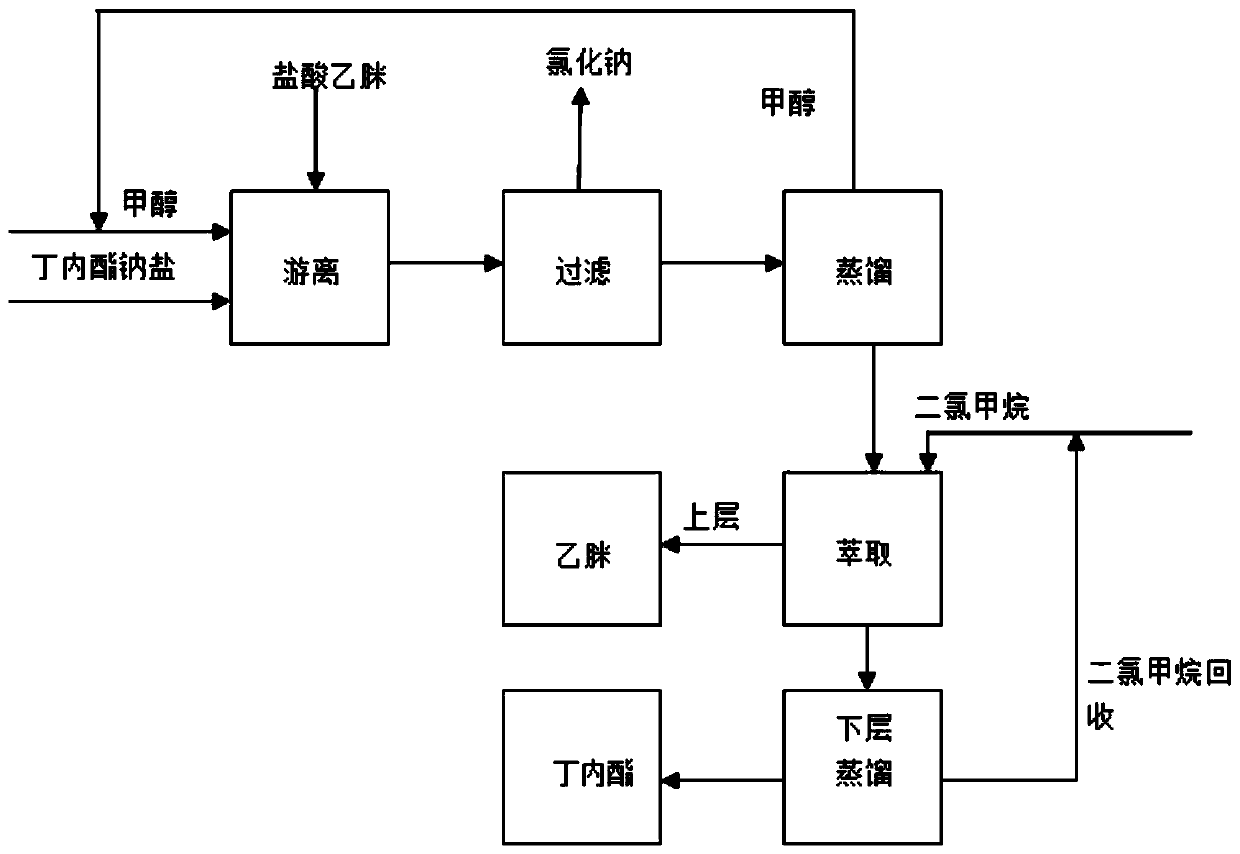
[0065] A kind of α-acetyl-γ-butyrolactone sodium salt free acetamidine hydrochloride process, the steps are as follows:
[0066] Add 73g of α-acetyl-γ-butyrolactone sodium salt and 50g of methanol into the three-necked flask, mix at room temperature, add 50g of acetamidine hydrochloride (content 92%), and control the temperature at 10-20°C for 2 hours;
[0067] After freeing, filter to obtain 28.5g of sodium chloride;
[0068] Control the temperature of the filtrate at 30-40°C for negative pressure distillation to recover methanol to obtain 94.5 g of distillate;
[0069] 120 g of dichloromethane was added to the distillate for extraction, and the dichloromethane was added twice in equal volumes to finally obtain 27.9 g of acetamidine liquid in the upper oil layer, and the yield of acetamidine was 98.8%;
[0070] The lower oil layer is dichloromethane and α-acetyl-γ-butyrolactone;
[0071] The lower oil layer was distilled at 50-60°C under normal pressure to recover dichlorom...
Embodiment 2
[0074] A kind of α-acetyl-γ-butyrolactone sodium salt free acetamidine hydrochloride process, the steps are as follows:
[0075] Add 73g of α-acetyl-γ-butyrolactone sodium salt and 50g of methanol into the three-necked flask, mix at room temperature, add 50g of acetamidine hydrochloride (content 92%), and control the temperature at 20-30°C for 2 hours;
[0076] After freeing, filter to obtain 26.2g of sodium chloride;
[0077] Control the temperature of the filtrate at 30-40°C for negative pressure distillation to recover methanol to obtain 96.5 g of distillate;
[0078] 120 g of dichloromethane was added to the distillate for extraction, and the dichloromethane was added twice in equal volumes to finally obtain 26.3 g of acetamidine liquid in the upper oil layer, and the yield of acetamidine was 93.13%;
[0079] The lower oil layer is dichloromethane and α-acetyl-γ-butyrolactone;
[0080] The lower oil layer was distilled at 50-60°C under normal pressure to recover dichloro...
Embodiment 3
[0083] A kind of α-acetyl-γ-butyrolactone sodium salt free acetamidine hydrochloride process, the steps are as follows:
[0084] Add 73g of α-acetyl-γ-butyrolactone sodium salt and 50g of methanol into the three-necked flask, mix at room temperature, add 50g of acetamidine hydrochloride (content 92%), and control the temperature at 30-40°C for 2 hours;
[0085] After freeing, filter to obtain 25.3g of sodium chloride;
[0086] Control the temperature of the filtrate at 30-40°C for negative pressure distillation to recover methanol and obtain 97.1 g of distillate;
[0087] 120 g of dichloromethane was added to the distillate for extraction, and the dichloromethane was added twice in equal volumes to finally obtain 25.3 g of acetamidine liquid in the upper oil layer, and the yield of acetamidine was 89.59%;
[0088] The lower oil layer is dichloromethane and α-acetyl-γ-butyrolactone;
[0089] The lower oil layer was distilled at 50-60°C under normal pressure to recover dichlor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com