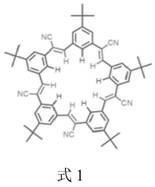
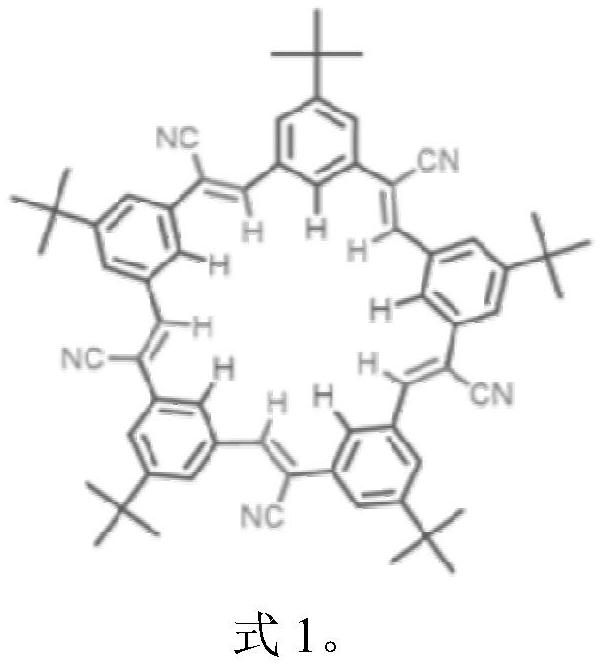
A kind of lithium-ion battery or lithium-sulfur battery electrolyte
A lithium-sulfur battery and electrolyte technology, applied in lithium batteries, secondary batteries, organic electrolytes, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] (1) Mix the cyclic carbonate solvent ethylene carbonate (EC) and the linear carbonate solvent diethyl carbonate (DEC) and ethyl methyl carbonate (EMC) according to the volume ratio of 1:1:1.
[0032] (2) At room temperature, the lithium salt LiPF 6 Dissolve the solvent obtained in step 1 so that the concentration is 1.0 mol / L, stir evenly, and add supramolecular CS as an additive at the same time.
[0033] (3) The mass percentage of the supramolecular CS in the lithium battery electrolyte is 0.1 wt%.
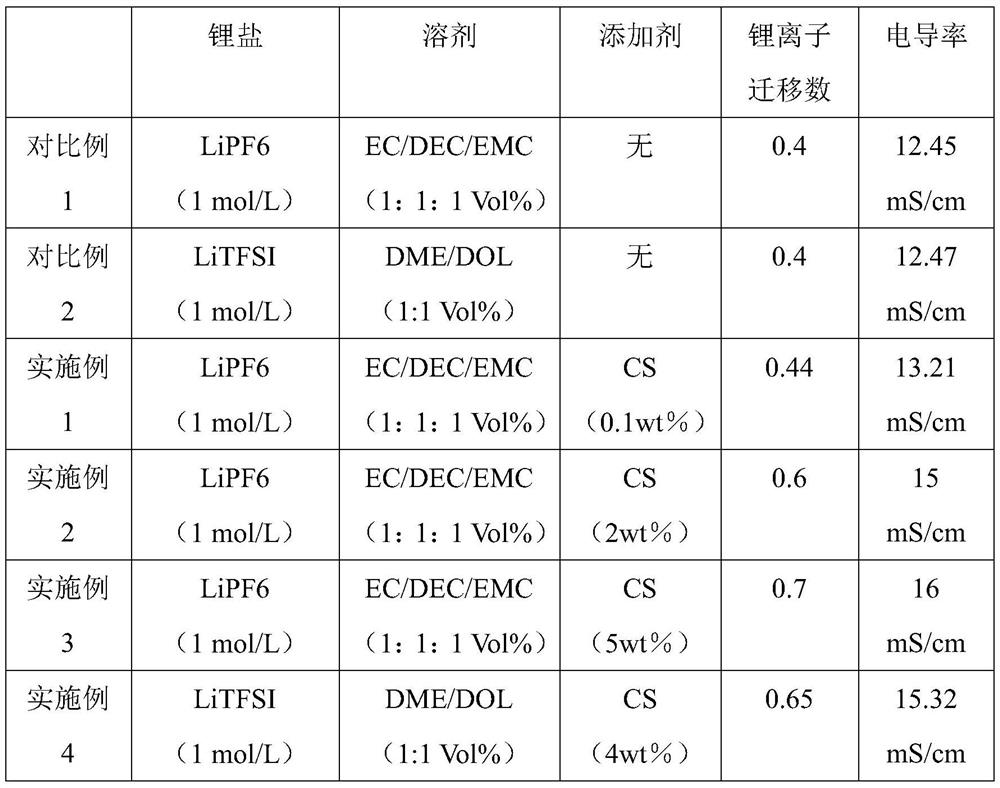
[0034] (4) Test the conductivity and lithium ion migration number of the electrolyte. The lithium ion migration number was 0.44. The conductivity was higher than that in Comparative Example 1, being 13.21 mS / cm.
Embodiment 2
[0036] (1) Mix the cyclic carbonate solvent ethylene carbonate (EC) and the linear carbonate solvent diethyl carbonate (DEC) and ethyl methyl carbonate (EMC) according to the volume ratio of 1:1:1.
[0037] (2) At room temperature, the lithium salt LiPF 6 Dissolve the solvent obtained in step 1 so that the concentration is 1.0 mol / L, stir evenly, and add supramolecular CS as an additive at the same time.
[0038] (3) The mass percentage of supramolecular CS in the electrolyte is 2wt%.
[0039] (4) Test the conductivity and lithium ion migration number of the electrolyte. The lithium ion migration number is 0.6. The conductivity was higher than that in Comparative Example 1, being 15 mS / cm.
Embodiment 3
[0041] (1) Mix the cyclic carbonate solvent ethylene carbonate (EC) and the linear carbonate solvent diethyl carbonate (DEC) and ethyl methyl carbonate (EMC) according to the volume ratio of 1:1:1.
[0042] (2) At room temperature, the lithium salt LiPF 6 Dissolve the solvent obtained in step 1 so that the concentration is 1.0 mol / L, stir evenly, and add supramolecular CS as an additive at the same time.
[0043] (3) The mass percentage of supramolecular CS in the electrolyte is 5wt%.
[0044] (4) Test the conductivity and lithium ion migration number of the electrolyte. The conductivity was higher than that in Comparative Example 1. The lithium ion migration number was 0.7. The conductivity was higher than that in Comparative Example 1, being 16 mS / cm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| electrical conductivity | aaaaa | aaaaa |
| electrical conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com