Kanggongyan tablet and its preparation process
A preparation process and anti-uterine inflammation technology, which is applied in the field of medicine, can solve the problems that the preparation process needs to be improved, the tablet stability of anti-uterine inflammation tablets is not ideal, and the degree of color change is serious, and the time to achieve drug absorption is shortened, Better tablet appearance and better product stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
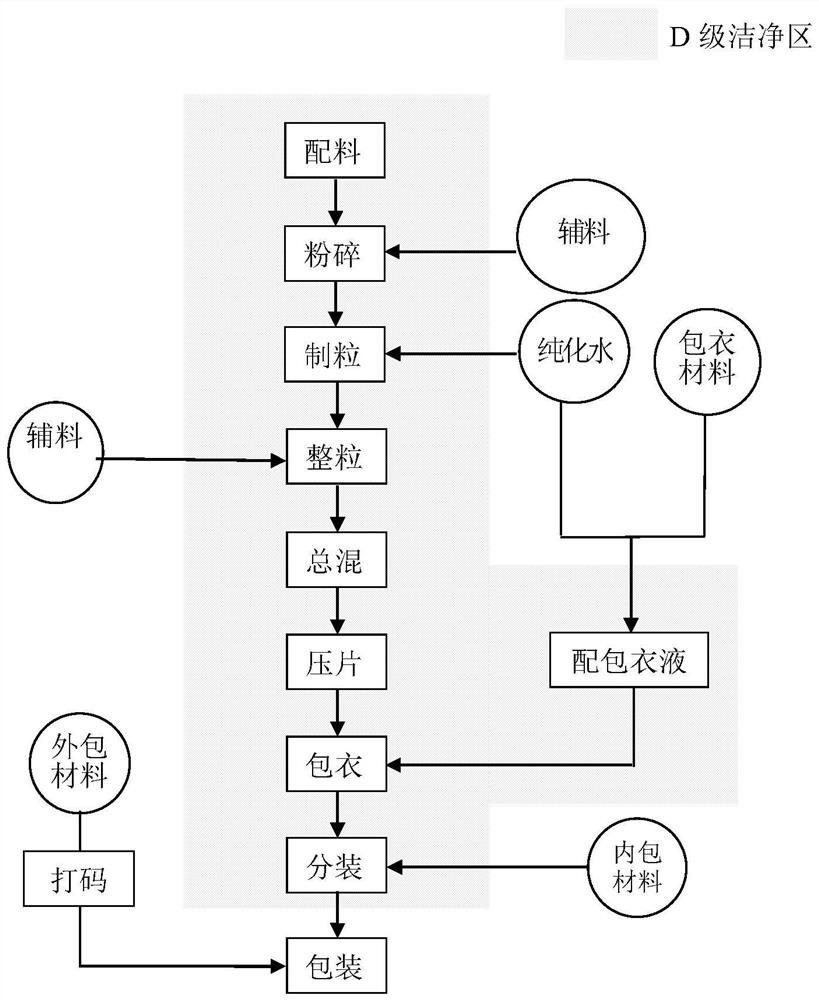
[0049] The preparation of the Kanggongyan Tablet of the present invention needs to be completed in a clean area of grade D or above, the temperature is kept at 18°C-26°C, and the relative humidity is controlled between 45%-65%. The number of air changes in the D-level clean area should be ≥15 times / h, the sedimentation bacteria should be ≤100cfu / 4 hours, the number of suspended particles under static conditions should not exceed 3520000 per cubic meter, and the particle size should not exceed 5.0um per cubic meter 29000.
[0050] The raw materials used in the examples of the present invention are common commercially available products.
Embodiment 1
[0051] Embodiment 1 A kind of prescription of Kanggongyan Tablet and its preparation method
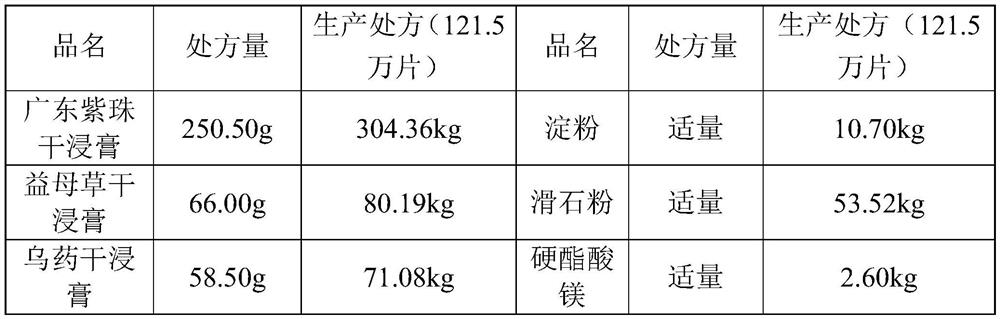
[0052] 1. Prescription
[0053] Prescription of anti-uterine tablets
[0054]
[0055] 2. Preparation process of Kanggongyan Tablets
[0056] A kind of preparation technology of Kanggongyan tablet comprises the following steps:
[0057] 1. Ingredients:
[0058] According to the production prescription, accurately weigh a batch of Guangdong Zizhu dry extract 304.36kg, Wuyao dry extract 71.08kg, Motherwort dry extract 80.19kg, starch 10.7kg, talc powder 53.52kg, put them in a clean stainless steel bucket, wait use.
[0059] 2. Crushing:
[0060] Mix and pulverize the Guangdong Zizhu dry extract, Wuyao dry extract, Leonurus dry extract, starch, and talcum powder handed over from the previous process into a fine powder, pass through a 100-mesh sieve, put it in a clean stainless steel bucket, and transfer it to the granulation process.
[0061] 3. Granulation:
[0062] The fine p...
Embodiment 2
[0069] Embodiment 2 A kind of prescription and preparation technology of Kanggongyan tablet
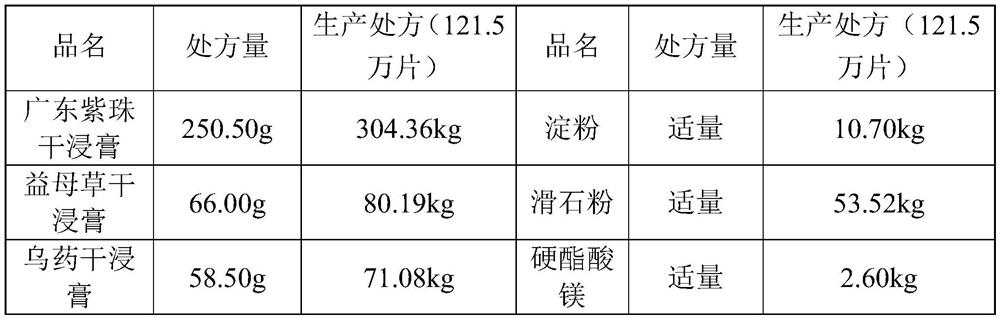
[0070] 1. Prescription
[0071] 1. Prescription of Kanggongyan Tablets
[0072]
[0073] 2. Prescription of film coating solution
[0074] product name 1.215 million tablets consumption Pharmaceutical film coating premixed excipients (stomach-soluble type) 2.0%~3.0% talcum powder 6~10kg purified water 70~90kg
[0075] 2. Preparation process
[0076] The preparation process of embodiment 2 is basically the same as that of embodiment 1, the difference is that, with reference to figure 1 The preparation process flow chart of embodiment 2, on the basis of steps 1 to 6 of embodiment 1, also increases the steps of film coating and packaging, specifically:
[0077] Steps 1-6: the same as steps 1-6 of the embodiment.
[0078] 7. Film coating
[0079] Receive qualified substrates, each batch is divided into four pots for film coating, each pot is ab...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com