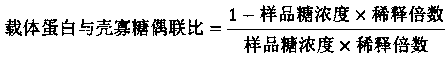Oligosaccharide vaccine for preventing invasive fungal infection
A technology for invasive fungi and vaccines, applied in the direction of antifungal agents, medical preparations with non-active ingredients, carrier-bound antigen/hapten components, etc., can solve the problem of lack of vaccines to prevent invasive fungal infections, and achieve broad-spectrum prevention Effects of fungal infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 Preparation of Chitooligosaccharides
[0033] 1.1
[0034] Dissolve 15g of chitosan with a degree of deacetylation of 1% in 300mL of 0.3M acetic acid, adjust the pH to 5.0 after the dissolution is complete, and add water to make the volume up to 500mL. Add 1.5g of chitosanase (130U / g) and stir in a water bath at 25-35°C for 8h. The chitosan enzymatic hydrolysate was filtered by plate and frame, and then ultrafiltration and nanofiltration with different pore size (400-5000Da) membranes were used to intercept the chitosan oligosaccharides ( 420 <0.1; 2% aqueous solution is centrifuged at 13000rpm for 2min without precipitation.
[0035] 1.2
[0036] Dissolve 18 g of chitosan with a degree of deacetylation of 95% in 300 mL of 0.2 M hydrochloric acid. After the dissolution is complete, adjust the pH to 6.5, and add water to make the volume to 500 mL. Add 1.5g of chitosanase (200U / g) and stir in a water bath at 25-35°C for 16h. The chitosan enzymatic hydrolysate is filt...
Embodiment 2
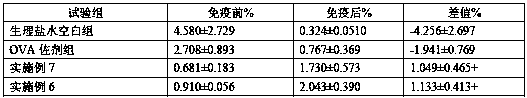
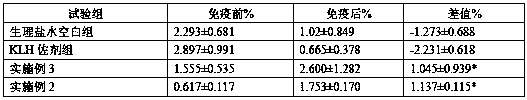
[0049] Example 2 Preparation of oligosaccharide vaccine
[0050] Take 50mg KLH dissolved in 1mL 0.1M sodium phosphate buffer pH7.2, containing 0.15M NaCl. Add 1 mg of chitosan with a degree of deacetylation of 10% and a molecular weight of 1000-3000Da, dissolve and mix. Take glutaraldehyde (CHO(CH 2 ) 3 CHO) 2μL of 50% aqueous solution, add the substrate solution, mix well, and keep it at 25°C with slight shaking for 60 minutes. Add 50mg of glycine solid and mix well, keep at 4℃ for 30min. Using 0.1M sodium phosphate buffer and 0.15M NaCl as the mobile phase, low molecular impurities were removed through Thermo Scientific™ Zeba™ SpinDesalting Columns to obtain the carrier protein solution coupled with chitosan oligosaccharide, which was placed in a 3000Da dialysis bag and dialyzed in pure water for 24h , Oligosaccharide vaccine is obtained after lyophilization.
Embodiment 3
[0051] Example 3 Preparation of oligosaccharide vaccine
[0052] Take 50mg KLH dissolved in 1mL 0.1M sodium phosphate buffer pH7.2, containing 0.15M NaCl. Add 1 mg of chitosan with a degree of deacetylation of 95% and a molecular weight of 1000-3000Da, dissolve and mix. Take glutaraldehyde (CHO(CH 2 ) 3 CHO) 11μL of 50% aqueous solution, add the substrate solution, mix well, and keep it at 25°C and shake it slightly for 60 minutes. Add 250mg of glycine solid and mix well, keep at 4℃ for 30min. Using 0.1M sodium phosphate buffer, containing 0.15M NaCl as the mobile phase, remove low-molecular impurities through Thermo Scientific™ Zeba™ SpinDesalting Columns to obtain the carrier protein solution coupled with chitosan oligosaccharide, and place it in a 2000Da dialysis bag with pure water for 24h dialysis , Oligosaccharide vaccine is obtained after lyophilization.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com