Acylhydrazone compound and preparation method and application thereof
A compound and lead compound technology, applied in organic chemistry, drug combination, pharmaceutical formulation, etc., can solve problems such as damage and unsatisfactory curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0040] The preparation method of the above-mentioned acylhydrazone compounds includes steps S110-S130.
[0041] The synthetic route of above-mentioned acylhydrazone compound is:
[0042]
[0043] The specific steps S110-S130 are as follows:
[0044] S110. Carrying out hydrazine hydrolysis reaction of compound A and 80% hydrazine hydrate by mass percentage to obtain compound B, wherein the structure of compound A is: The structural formula of compound B is:
[0045] Specifically, compound A was prepared using isoniazid. The specific steps for preparing compound A include the operations of S111-S115.
[0046] S111. Compound D is prepared.
[0047] Isoniazid and CS 2 Nucleophilic addition reaction in KOH affords compound D. The reaction formula for preparing compound D is as follows:
[0048]
[0049] Specifically, first dissolve the KOH solid in absolute ethanol to obtain a potassium hydroxide ethanol solution, and then add isoniazid to the potassium hydroxide et...
Embodiment 1
[0079] (1) Synthesis of Compound C
[0080] The first step: the synthetic route of compound D is as follows:
[0081]
[0082] Mix 7.8016g (0.1390mol) of KOH solid with 150ml of absolute ethanol, heat and stir until it dissolves, and after the solution is cooled to room temperature, add 10.0380g (0.0732mol) of isoniazid to it, and slowly add 6mL of CS 2 , magnetically stirred at room temperature for 10 h to produce a yellow solid, which was then suction filtered and dried in vacuo to obtain Compound D as a yellow powder. Yield: 19.4775 g, crude yield: 105.9%.
[0083] The second step: the synthetic route of compound C is as follows:
[0084]
[0085] 12.9884g (0.0517mol) of compound D obtained in the first step and 20mL (0.41mol) of 80% hydrazine hydrate in mass percentage were mixed with 20mL of ice water after magnetic stirring and reflux in an oil bath at 143°C for 4 hours, and then Adjust the pH to 5 with concentrated hydrochloric acid to produce a white solid, th...
Embodiment 2
[0103] (1) Synthesize Compound B according to the steps of Synthesis Compound B in Example 1.
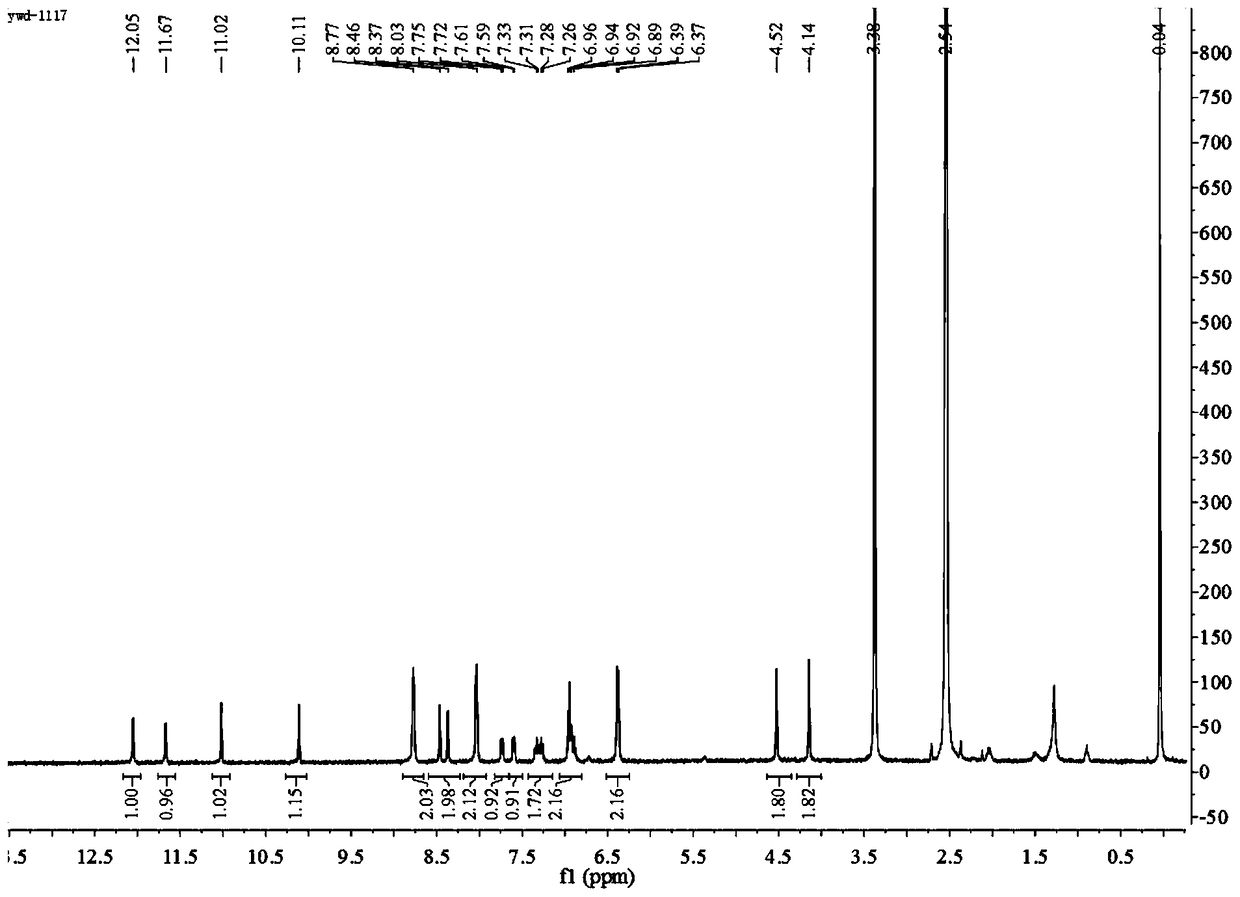
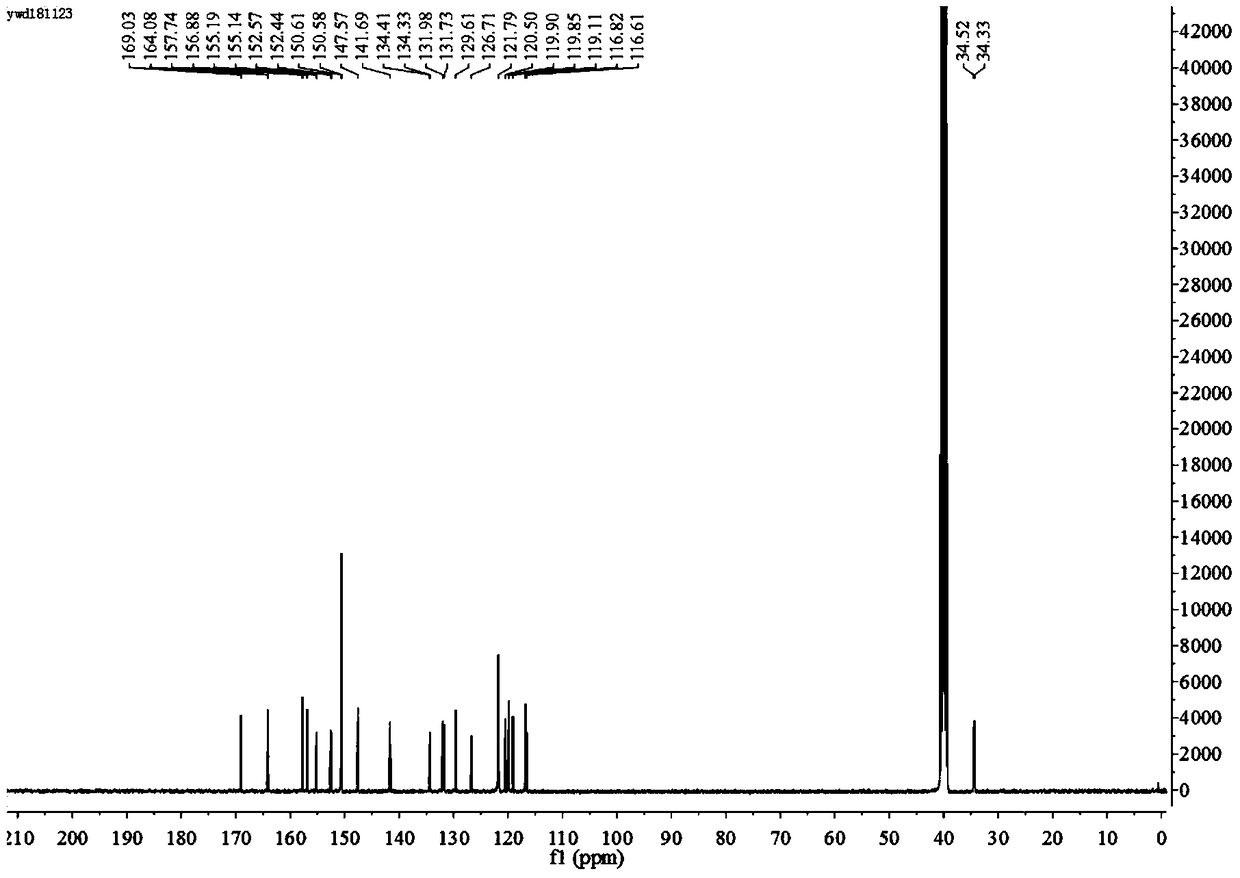
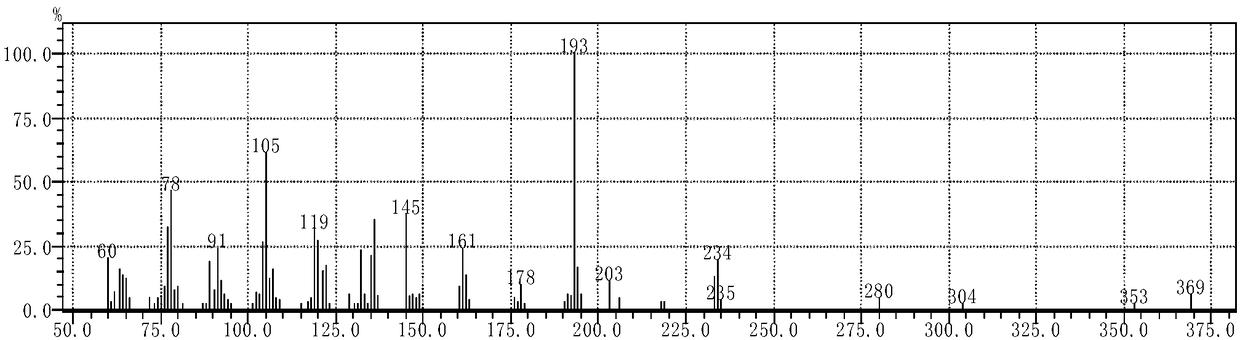
[0104] (2) Dissolve 0.2085 g (0.78 mmol) of compound B in 70 mL of anhydrous methanol to obtain a pre-reaction solution. Then, 0.18 mL (1.43 mmol) of cinnamaldehyde was added to the pre-reaction solution, and heated to reflux at 67° C. for 4 h. Stand to cool and crystallize, filter with suction, and recrystallize from absolute ethanol to obtain 0.2982g of white crystals, which have the structural formula: acylhydrazone compounds. The yield was 38.1%. MS (ESI) m / z: 380.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com