Method for selectively separating lithium from leaching solution of cathode material for waste lithium-ion batteries
A technology for lithium-ion batteries and positive electrode materials, which is applied in the field of regeneration of useful parts of waste batteries, can solve the problems of low recovery rate, unstable properties of lithium-ion sieve adsorbent, and weak selectivity, and achieve low solubility and high precipitation rate. The effect of high and low cost of use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
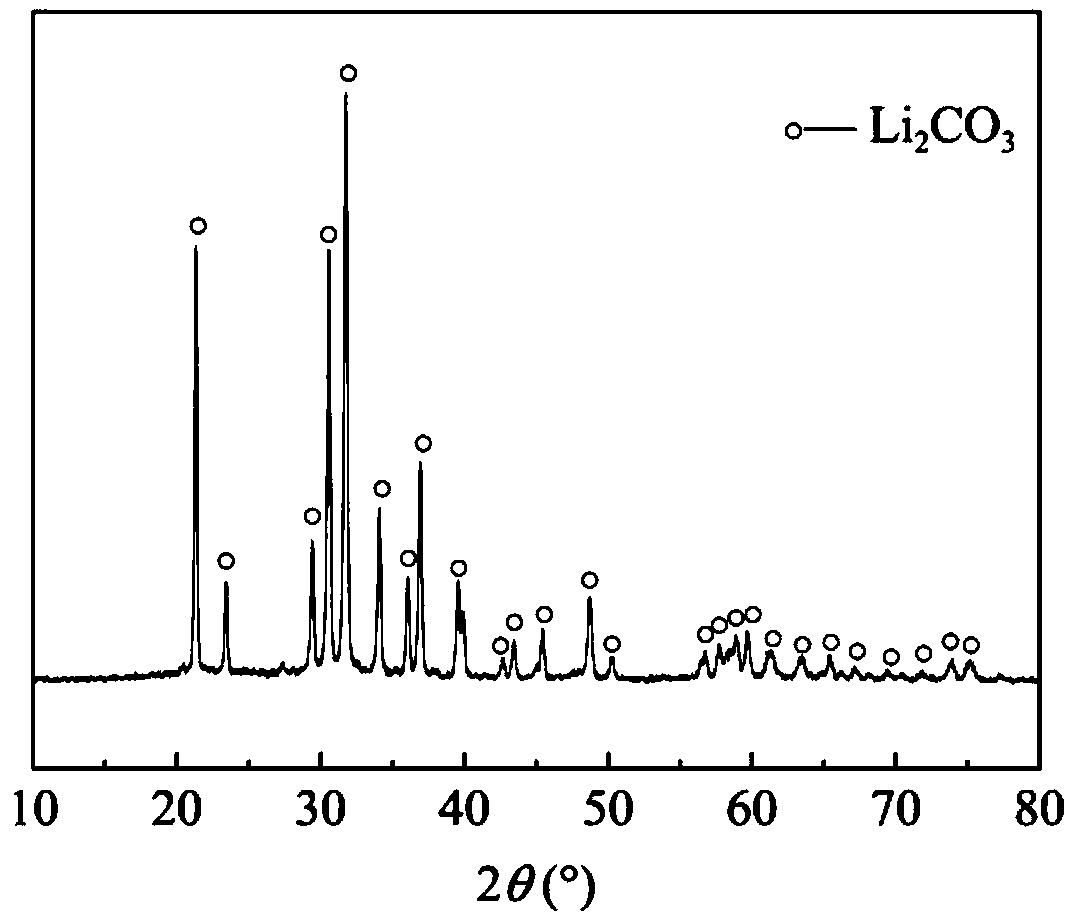
[0037] The sulfuric acid leaching solution of the positive electrode material of the waste lithium-ion battery is used as the raw material, the pH value is 6, and the composition is Li 0.5g / L, Co 4.2g / L. A certain amount of benzo15-crown-5 was dissolved in sulfonated kerosene to prepare an extracted organic phase with a mass fraction of crown ether of 10%. A single extraction was performed at a temperature of 80°C and a phase ratio of 2:1, and the extraction time was 10 minutes. After the organic phase was separated from the aqueous phase, the temperature was 25°C, and the volume ratio of the saturated sodium carbonate solution to the organic phase was 2:1, and the volume ratio of the saturated sodium carbonate solution to the organic phase was back-extracted for 10 minutes to obtain a white lithium carbonate precipitate, and the recovery rate of lithium reached 95.3%. Such as figure 2 Shown is the XRD spectrum of gained lithium carbonate, as can be seen from the figure, whe...
Embodiment 2
[0039] The hydrochloric acid leaching solution of the positive electrode material of the waste lithium ion battery is used as the raw material, the hydrogen ion concentration is 6mol / L, and the composition is Li 8.3g / L, Co 22.5g / L, Ni 23.8g / L, Mn 21.3g / L. A certain amount of 4-aminobenzo15-crown-5 was dissolved in sulfonated kerosene to prepare an extracted organic phase with a crown ether mass fraction of 30%. At a temperature of 25°C and a ratio of 1:5, a 10-stage cross-flow extraction was carried out, and the extraction time was 60 minutes. After the organic phase was separated from the aqueous phase, the temperature was 95°C, and the volume ratio of the saturated sodium carbonate solution to the organic phase was 2:1, and the volume ratio of the saturated sodium carbonate solution to the organic phase was back-extracted for 60 minutes to obtain a white lithium carbonate precipitate, and the recovery rate of lithium reached 98.1%.
Embodiment 3
[0041] The sulfuric acid leaching solution of the positive electrode material of the waste lithium-ion battery is used as the raw material, the pH value is 1, the composition is Li 5.3g / L, and Mn 80g / L. A certain amount of 4-hydroxybenzo15-crown-5 was dissolved in dichloromethane to prepare an extracted organic phase with a mass fraction of crown ether of 15%. At a temperature of 30°C, a 10-stage countercurrent extraction was carried out under the condition of ratio 2:1, and the extraction time was 45 minutes. After the organic phase was separated from the aqueous phase, the temperature was 25°C, and the volume ratio of the saturated sodium carbonate solution to the organic phase was 1:2, and the volume ratio of the saturated sodium carbonate solution to the organic phase was back-extracted for 50 minutes to obtain a white lithium carbonate precipitate, and the recovery rate of lithium reached 96.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com