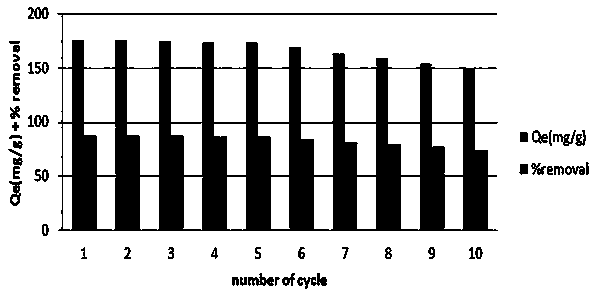
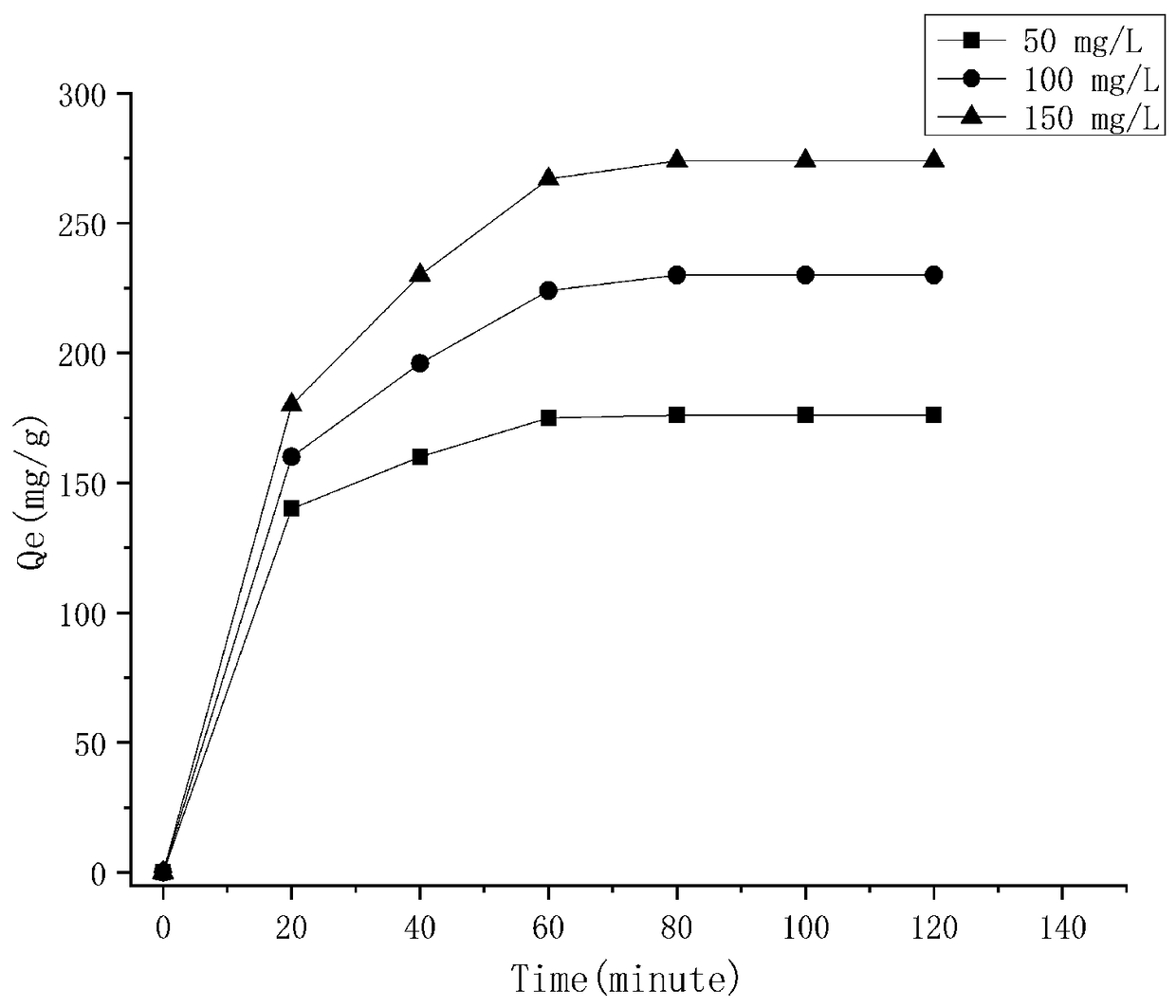
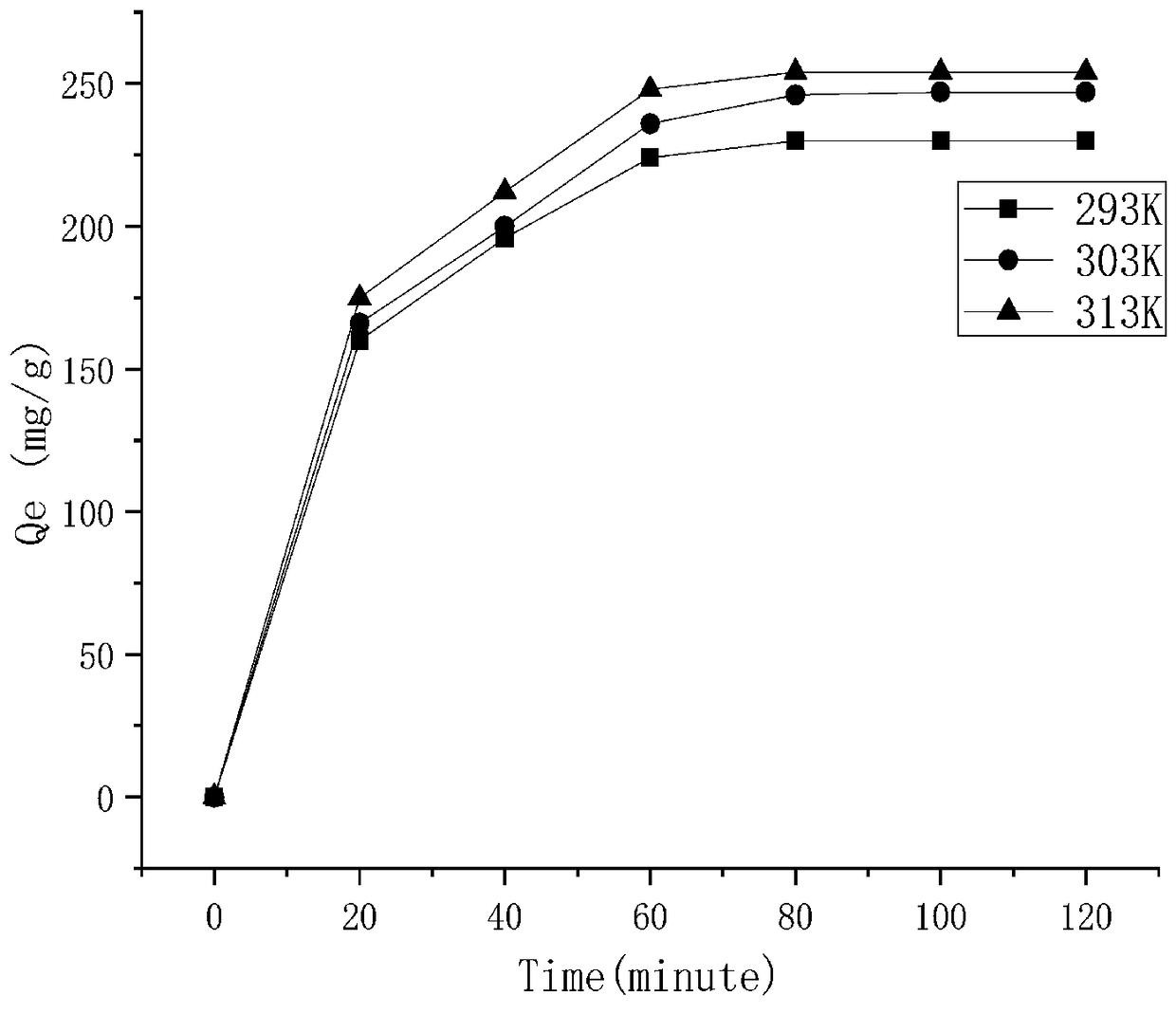
COFs (Covalent Organic Frameworks) nano adsorbing material with high specific surface area, preparation method and application
A nanomaterial and reaction technology, applied in the field of COFs materials and their preparation, can solve the problems of poor adsorption performance of methyl violet dye and polycyclic aromatic hydrocarbons, and achieve the effect of efficient adsorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1: Preparation of COFs nanomaterials
[0037]Take 63.0 mg trialdehyde phloroglucinol and 75.6 mg melamine in a glass vial; add 3 mL of dimethyl sulfoxide and 3 mL of methanol (1:1) and 0.2 mL of 6 mol / L acetic acid, sonicated in a glass vial for 10 min, and the mixture was sealed in a Teflon-lined autoclave and heated at 120 °C for 24 h. After cooling to room temperature, a red powder was obtained by filtration, washing with tetrahydrofuran, DMSO and ethanol several times, and drying in a vacuum oven. Take 2.5 mg of adsorbent in a 20 mL glass bottle, add 10 mL of 150 mg / L methyl violet dye solution at a temperature of 313K, stir at 160 rpm, and adsorb for 60 min. The adsorption capacity was 274 mg / g.
Embodiment 2
[0038] Example 2: Take 63.0 mg trialdehyde phloroglucinol and 56.7 mg melamine in a glass vial; add 3 mL of dimethyl sulfoxide and 3 mL of methanol (1:1) and 0.2 mL of 6 mol / L acetic acid, sonicated in a glass vial for 10 min, and the mixture was sealed in a Teflon-lined autoclave and heated at 120 °C for 28 h. After cooling to room temperature, a red powder was obtained by filtration, washing with tetrahydrofuran, DMSO and ethanol several times, and drying in a vacuum oven. Take 2.5 mg of adsorbent and place it in a 20 mL glass bottle, add 10 mL of 100 mg / L methyl violet dye solution with a volume of 10 mL, the temperature is 313K, the stirring speed is 160 rpm, and the adsorption time is 60 min. The adsorption capacity was 247 mg / g.
Embodiment 3
[0039] Example 3: Take 60.6 mg trialdehyde phloroglucinol and 37.8 mg melamine in a glass vial; add 3 mL of dimethyl sulfoxide and 3 mL of methanol (1:1) and 0.2 mL of 6 mol / L acetic acid, sonicated in a glass vial for 10 min, and the mixture was sealed in a Teflon-lined autoclave and heated at 120 °C for 36 h. After cooling to room temperature, a red powder was obtained by filtration, washing with tetrahydrofuran, DMSO and ethanol several times, and drying in a vacuum oven. Take 2.5 mg of adsorbent and place it in a 20 mL glass bottle, add 10 mL of 100 mg / L methyl violet dye solution with a volume of 10 mL, the temperature is 313K, the stirring speed is 160 rpm, and the adsorption time is 60 min. The adsorption capacity was 221 mg / g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com