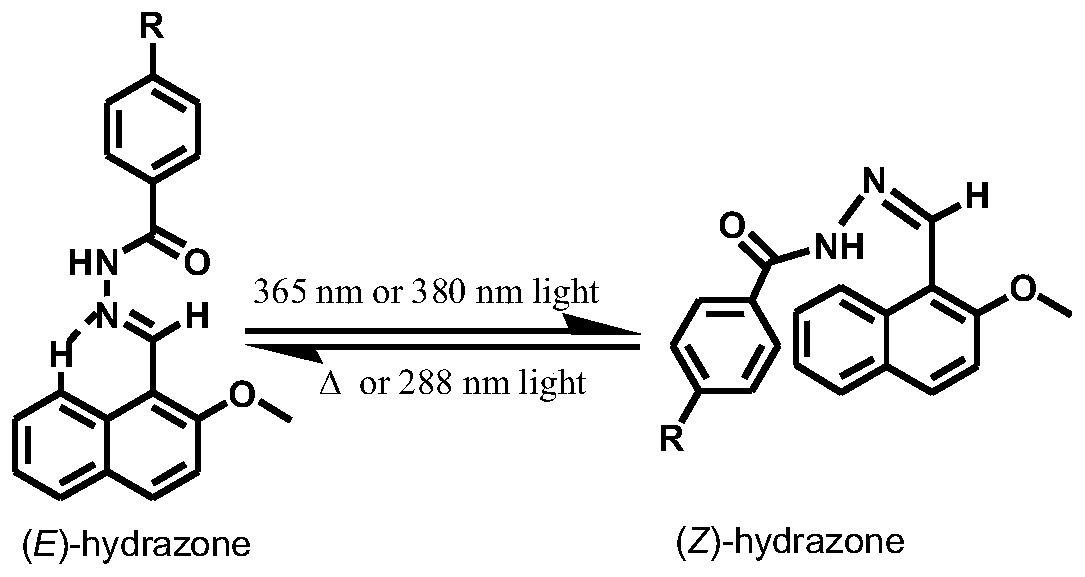
A kind of acylhydrazone molecular switch, its preparation method and application
A technology of compound and general formula, which is applied in the field of acylhydrazone molecular switch and its preparation, can solve the problems of low E-Z conversion rate, no reports of loading and controlled release, low photo-induced E-Z conversion rate, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Molecular switch I (in general formula (I), R 1 =R 2 =R 3 =-(OCH 2 CH 2 ) 5 OH; R 4 =CH 3 ; 5 = H; R 6 =-OCH 2 (CH 2 ) 12 CH 3 )Synthesis:
[0059]
[0060] A compound of formula (II) (0.2-2 g) and ethanol (10-50 mL) were added to a round bottom flask, followed by a solution of compound of formula (III) (0.3-5 g) in ethanol (10-50 mL). After heating and stirring at 40-80°C for 1-10min, filter. The collected solids were washed with ethanol to obtain the compound of formula (I) in greater than 95% yield.
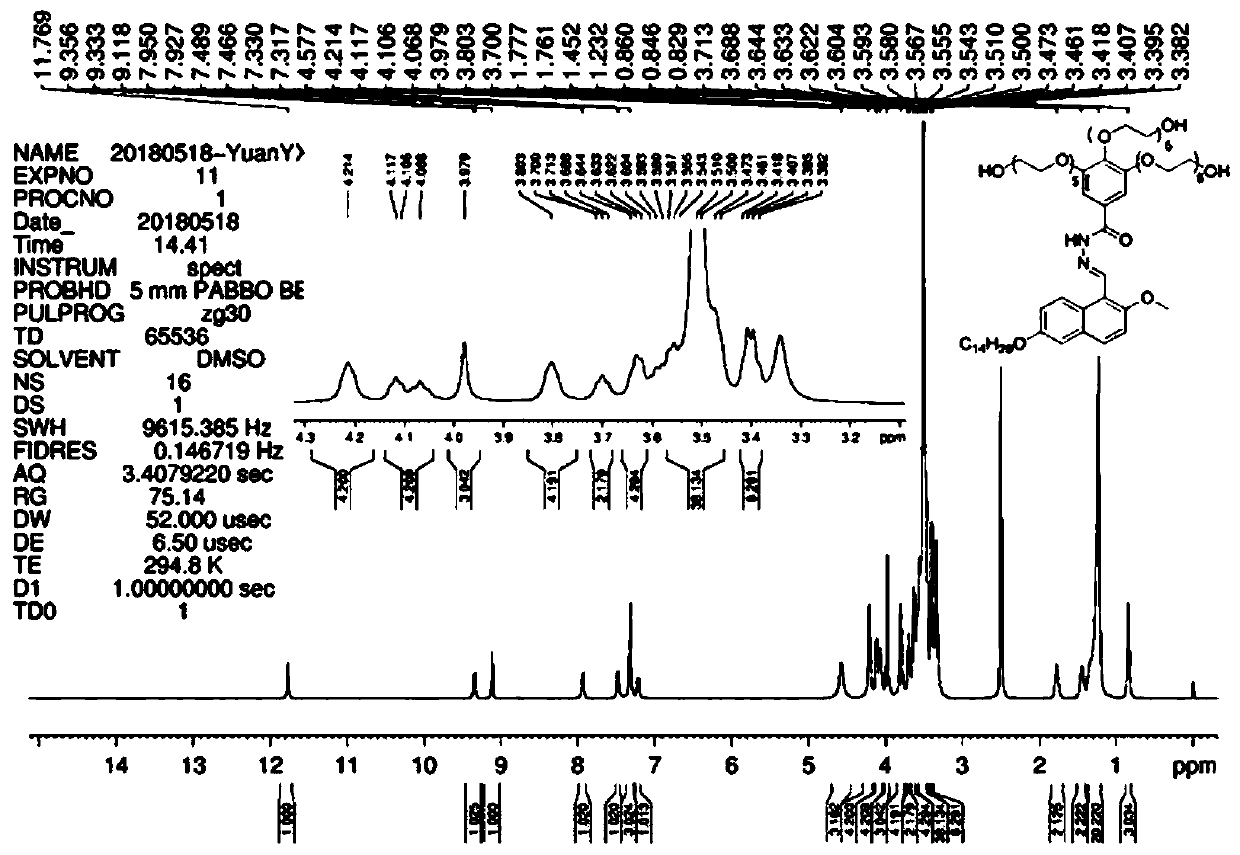
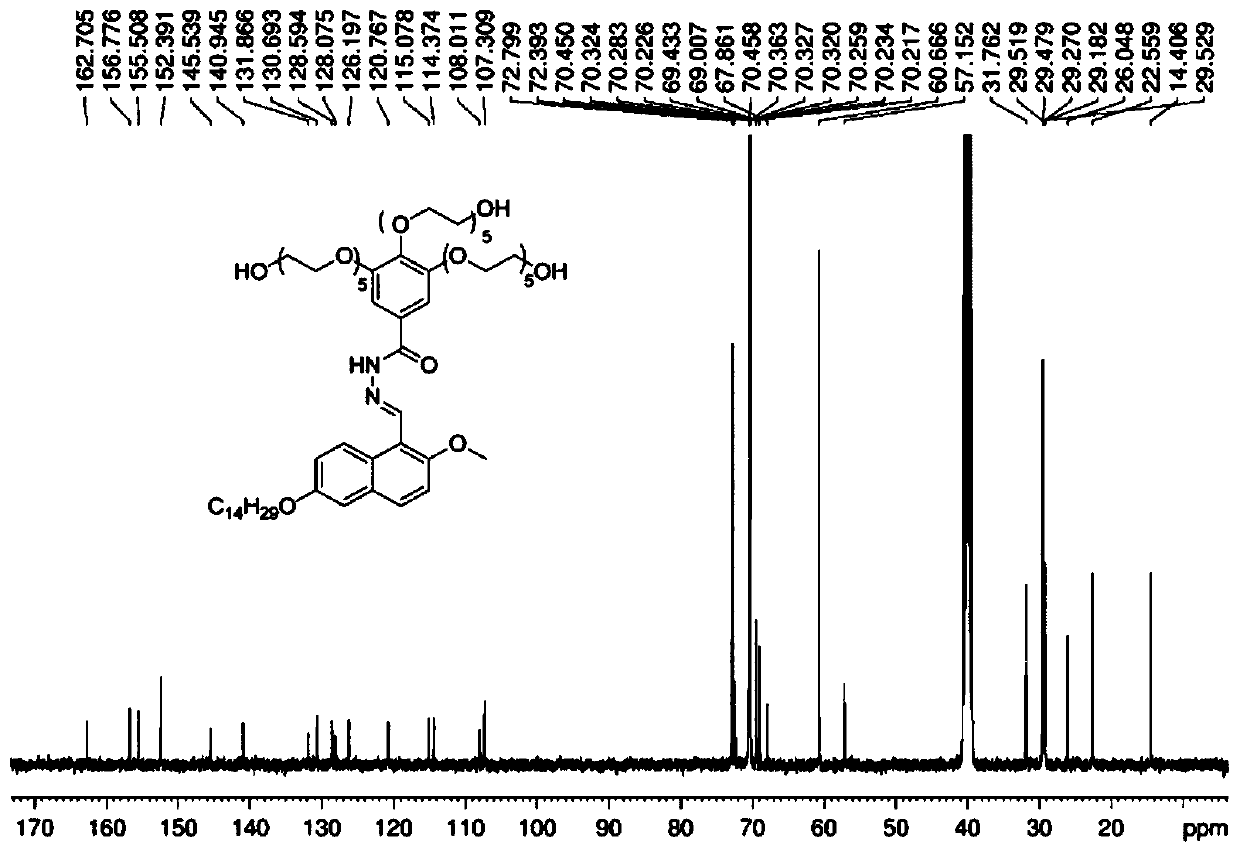
[0061] Formula (I) compound in DMSO-d6 1 H-NMR spectrum see figure 2 , 13 C-NMR spectrum see image 3 , HRMS spectrum see Figure 4 .
Embodiment 2
[0063] The compound of formula I (in general formula (I), R 1 =R 2 =R 3 =-(OCH 2 CH 2 ) 5 OH; R 4 =CH 3 ; 5 = H; R 6 =-OCH 2 (CH 2 ) 12 CH 3 ) was dissolved in water, and the obtained aqueous solution was irradiated with 365nm light of a portable ultraviolet lamp to measure the 1 H-NMR spectrum, 90% of the E-isomer was converted to the Z-isomer. its in D 2 Before and after illumination in O 1 H-NMR spectrum, see Figure 5 .
Embodiment 3
[0065] Compounds of formula (I) (in general formula I, R 1 =R 2 =R 3 =-(OCH 2 CH 2 ) 5 OH; R 4 =CH 3 ; 5 = H; R 6 =-OCH 2 (CH 2 ) 12 CH 3 ) is dissolved in DMSO, with 388nm ultraviolet light for different time, measure the ultraviolet-visible spectrum of different light time, see Figure 6 (a); It is found that the absorption band at 377nm gradually weakens, while the absorption band at 273nm gradually increases, see Figure 6 (b).
[0066] Figure 6 is a compound of formula (I) (in general formula I, R 1 =R 2 =R 3 =-(OCH 2 CH 2 ) 5 OH; R 4 =CH 3 ; 5 = H; R 6 =-OCH 2 (CH 2 ) 12 CH 3 ) UV-Vis spectra in DMSO as a function of illumination time (a) and absorbance at 377nm and 273nm as a function of illumination time (b). [Formula I]=6.0×10 -5 M, light source 388nm light.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com