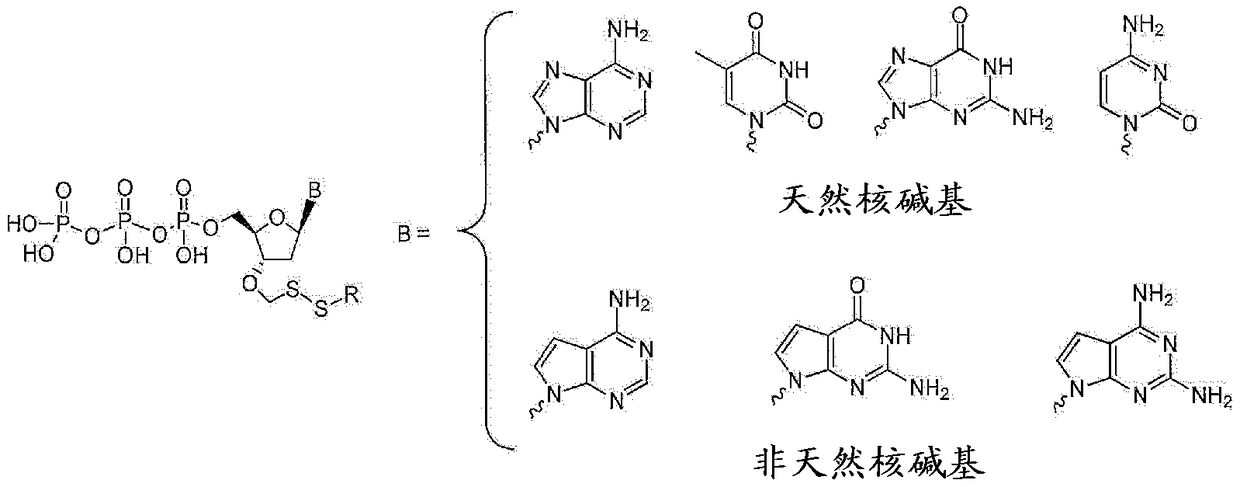
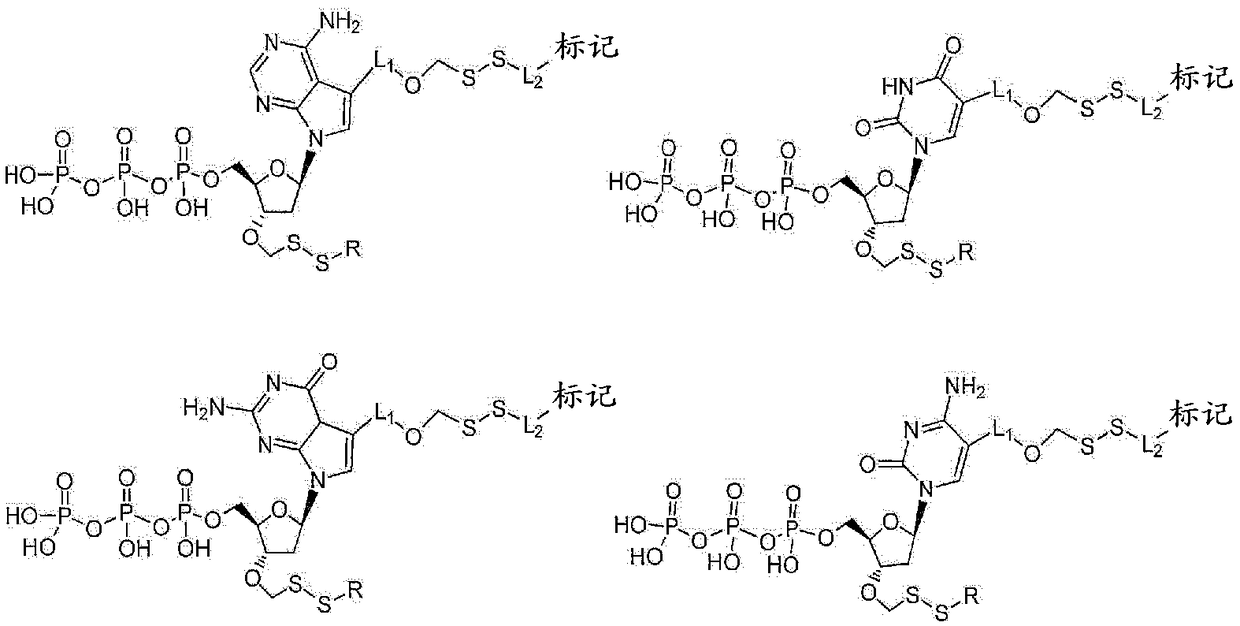
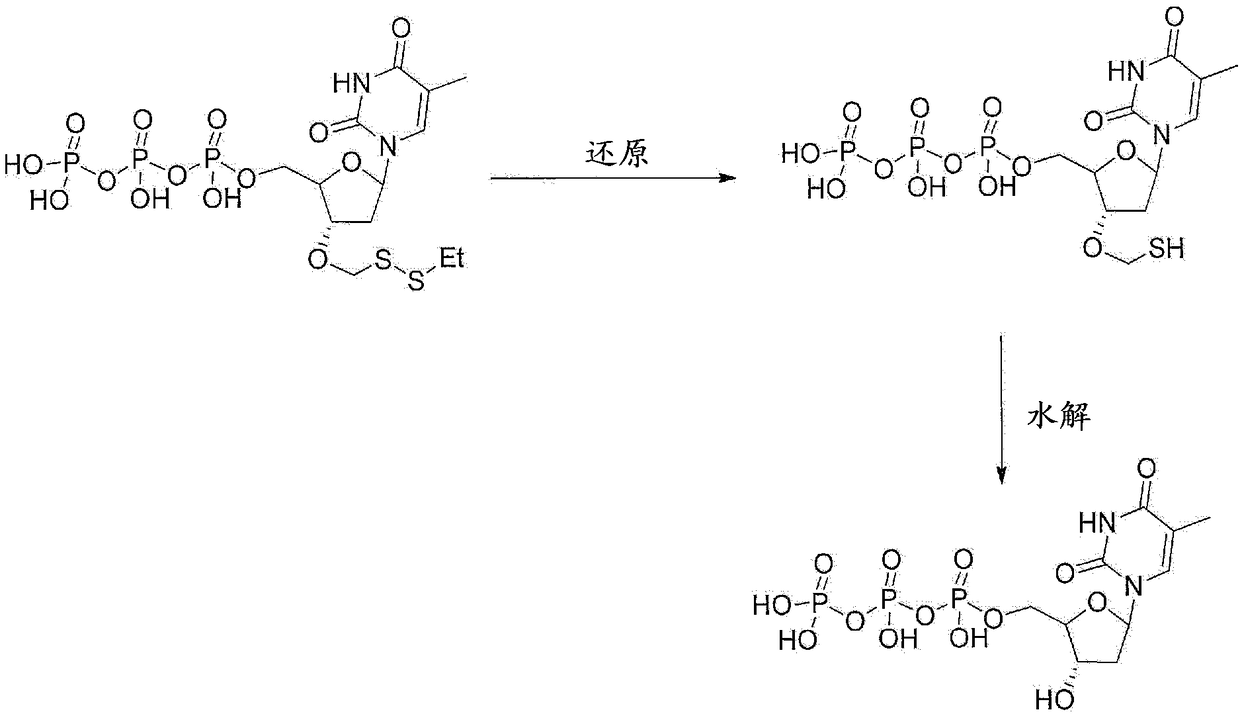
Nucleotide analogues
A technology for deoxynucleoside triphosphates and compounds, which is applied in the field of nucleotide analogs, and can solve the problems of no disclosure of synthetic methods, no disclosure of deprotection conditions and enzymatic binding data, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0288] Synthesis of 3'-O-(methylthiomethyl)-5'-O-(tert-butyldimethylsilyl)-2'-deoxythymidine (2)
[0289] In a 250 mL round bottom flask, 5'-O-(tert-butyldimethylsilyl)-2'-deoxythymidine (1) (2.0 g, 5.6 mmol) was dissolved in DMSO (10.5 mL), acetic acid (4.8 mL) and acetic anhydride (15.4 mL) were stirred at room temperature for 48 hours. Then saturate by adding K 2 CO 3 solution quenches the mixture until gaseous CO 2 Release stops. The mixture was then extracted with EtOAc (3X100 mL) using a separatory funnel. Then with saturated NaHCO 3 solution (2X150mL), the combined organic extracts should be washed with a distribution funnel, washed with Na 2 SO 4 Dry the organic layer. The organic portion was concentrated by rotary evaporation. The reaction mixture was finally purified by silica gel column chromatography (Hex:EtOAc / 7:3-1:1), see Figure 8. 3'-O-(methylthiomethyl)-5'-O-(tert-butyldimethylsilyl)-2'-deoxythymidine (2) was obtained as white powder in 75% yield (...
Embodiment 2
[0291] Synthesis of 3'-O-(ethyldithiomethyl)-2'-deoxythymidine (4)
[0292] Dissolve in 20 mL of anhydrous CH 2 Cl 2 Add Et to compound 2 (1.75g, 4.08mmol) in 3 N (0.54 mL, 3.87 mmol) and 5.0 g molecular sieve-3A were stirred under Ar atmosphere for 30 minutes. The reaction flask was then placed on an ice bath to bring the temperature below zero and 1.8eq 1M SO was slowly added 2 Cl 2 CH 2 Cl 2 The solution (1.8 mL) was stirred at the same temperature for 1.0 hr. The ice bath was then removed to allow the flask to come to room temperature and a solution of potassium thiotosylate (1.5 g) in 4 mL of anhydrous DMF was added and stirred at room temperature for 0.5 hours.
[0293] Then 2eq EtSH (0.6mL) was added and stirred for another 40 minutes. Then with 50mL CH 2 Cl 2 The mixture was diluted and filtered through Celite-S in a funnel. with sufficient amount of CH 2 Cl 2 Samples were washed to ensure product was filtered out. Then concentrate CH 2 Cl 2 The extract...
Embodiment 3
[0296] Synthesis of 3'-O-(ethyldithiomethyl)-2'-deoxythymidine triphosphate (5)
[0297] To compound 4 (0.268 g, 0.769 mmol) was added proton sponge (210 mg) equipped with a rubber stopper in a 25 mL flask. The samples were dried overnight under high vacuum. This material was dissolved in 2.6 mL (MeO) under argon atmosphere 3 PO. Place the flask equipped with an Ar-gas supply on an ice bath and stir until the temperature reaches subzero. Then immediately add 1.5 equivalents of POCl with a syringe 3 And stirred at the same temperature under an argon atmosphere for 2 hours. The ice bath was then removed to prepare a mixture of tributylammonium-pyrophosphate (1.6 g) and Bu 3 A mixture of N (1.45 mL) in anhydrous DMF (6 mL). The whole mixture was added at once and stirred for 10 minutes. The reaction mixture was then diluted with TEAB buffer (30 mL, 100 mM) and stirred at room temperature for an additional 3 hours. The crude product was concentrated by rotary evaporation a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com