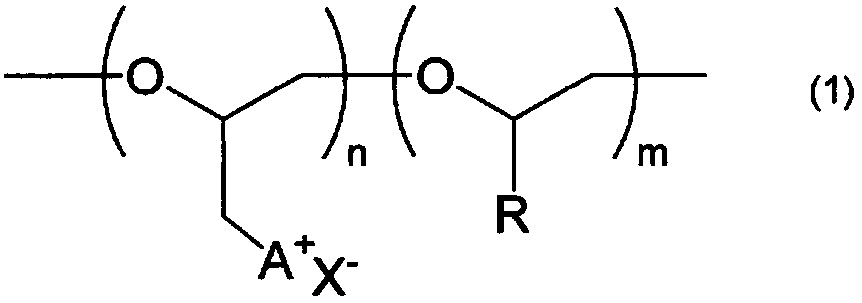
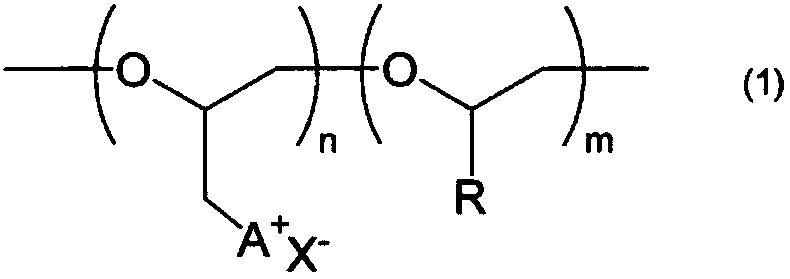
Ionic composition and crosslinked product
A composition and ionic technology, applied in the field of ionic compositions, can solve the problems of inability to use at low temperature, insufficient low temperature characteristics, insufficient inhibition effect, etc., and achieve the effect of flammability inhibition and excellent low temperature characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
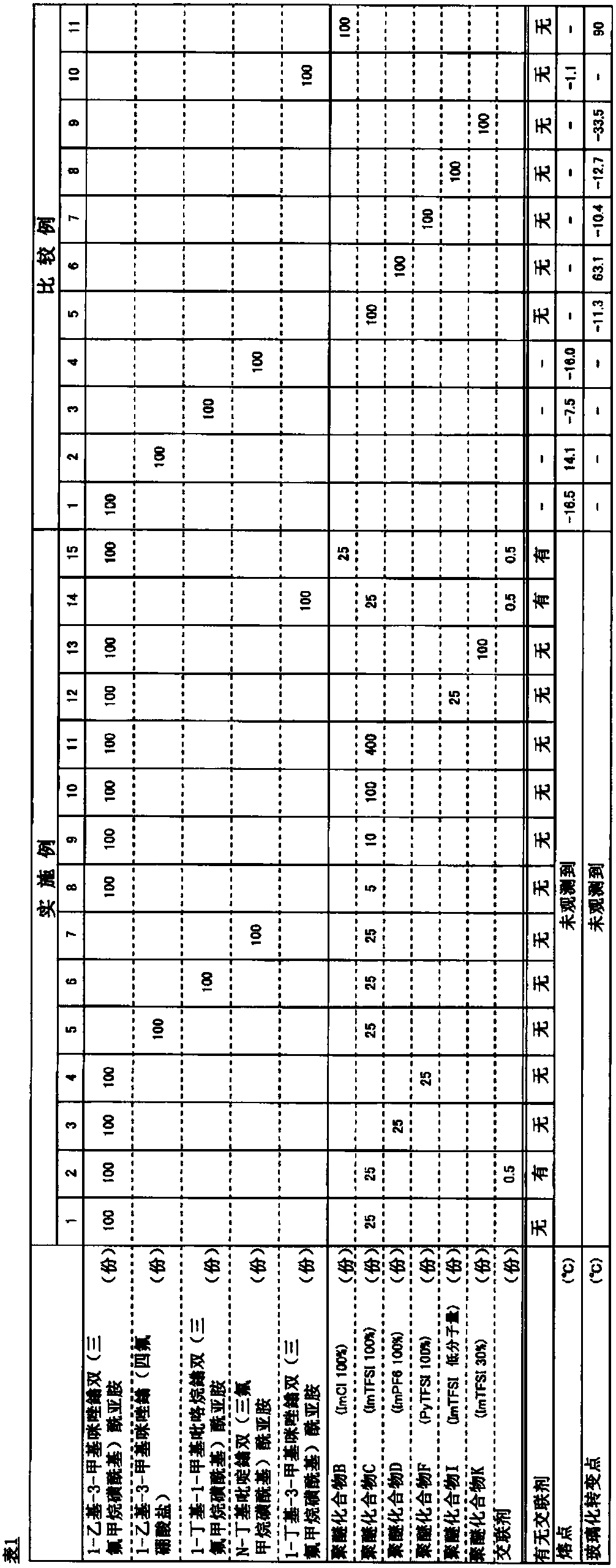
[0083] Hereinafter, the present invention will be described in more detail based on examples, but the present invention is not limited to these examples. In addition, "part" and "%" in the following are based on weight unless otherwise indicated. In addition, tests and evaluations were as described.
[0084] [Number average molecular weight (Mn) and molecular weight distribution (Mw / Mn)]
[0085] (1) The number-average molecular weight (Mn) and molecular weight distribution (Mw / Mn) of the base polymer (polyether compound having no cationic group) were determined as polyphenylene by gel permeation chromatography (GPC) using tetrahydrofuran as a solvent. Measured in terms of ethylene conversion value. In addition, HLC-8320 (manufactured by TOSOH CORPORATION) was used as a measuring device, four TSKgel SuperMultiporeHZ-H (manufactured by TOSOH CORPORATION) connected in series was used as a column, and differential refractometer RI-8320 (manufactured by TOSOH CORPORATION) was us...
manufacture example 1
[0094] (Living anionic copolymerization of epihalohydrin and glycidyl methacrylate)
[0095] Into a glass reactor with a stirrer replaced with argon, 0.032 g of tetra-n-butylammonium bromide and 5 ml of toluene were added, followed by cooling to 0°C. Next, a solution obtained by dissolving 0.029 g (2.5 equivalents to tetra-n-butylammonium bromide) of triethylaluminum in 0.25 ml of n-hexane was added and reacted for 15 minutes to obtain a catalyst composition. To the obtained catalyst composition, 9.5 g of epichlorohydrin and 0.5 g of glycidyl methacrylate were added, and polymerization reaction was performed at 0°C. After the polymerization reaction started, the viscosity of the solution gradually increased. After reacting for 1 hour, a small amount of 2-propanol was added to the polymerization reaction solution to terminate the reaction. Next, the obtained polymerization reaction solution was diluted with toluene, and poured into 2-propanol to obtain 8.3 g of a white rubber...
manufacture example 2
[0097] (The epichlorohydrin unit in the polyether compound A is based on the quaternization of 1-methylimidazole)
[0098] 8.0 g of polyether compound A obtained in Production Example 1, 22.0 g of 1-methylimidazole, and 16.0 g of N,N-dimethylformamide were added to a glass reactor with a stirrer replaced with argon. In a container, heat to 80°C. After reacting at 80° C. for 144 hours, the reaction was terminated by cooling to room temperature, and a part of the obtained reaction solution was extracted and dried under reduced pressure at 50° C. for 120 hours to obtain 15.0 g of a reddish-brown resinous substance. the resinous substance 1 As a result of H-NMR measurement and elemental analysis, it was confirmed that it was a polyether compound B in which all the chlorine groups in the epichlorohydrin units in the polyether compound A obtained in Production Example 1 of the starting material were substituted with Polyether compound B having a 1-methylimidazolium halide group in...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com