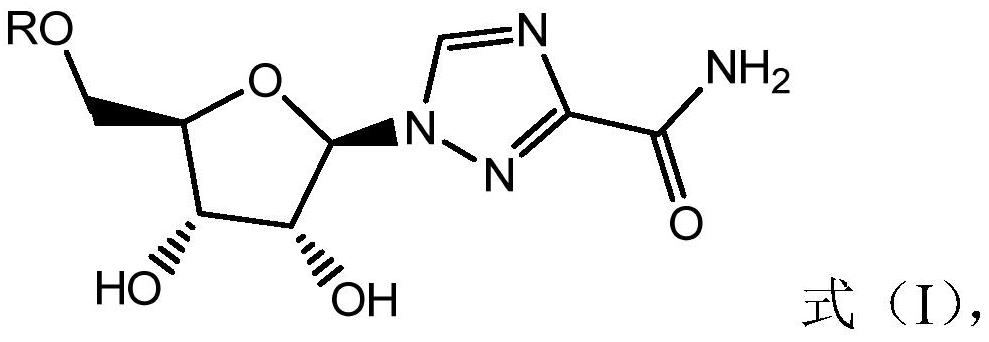
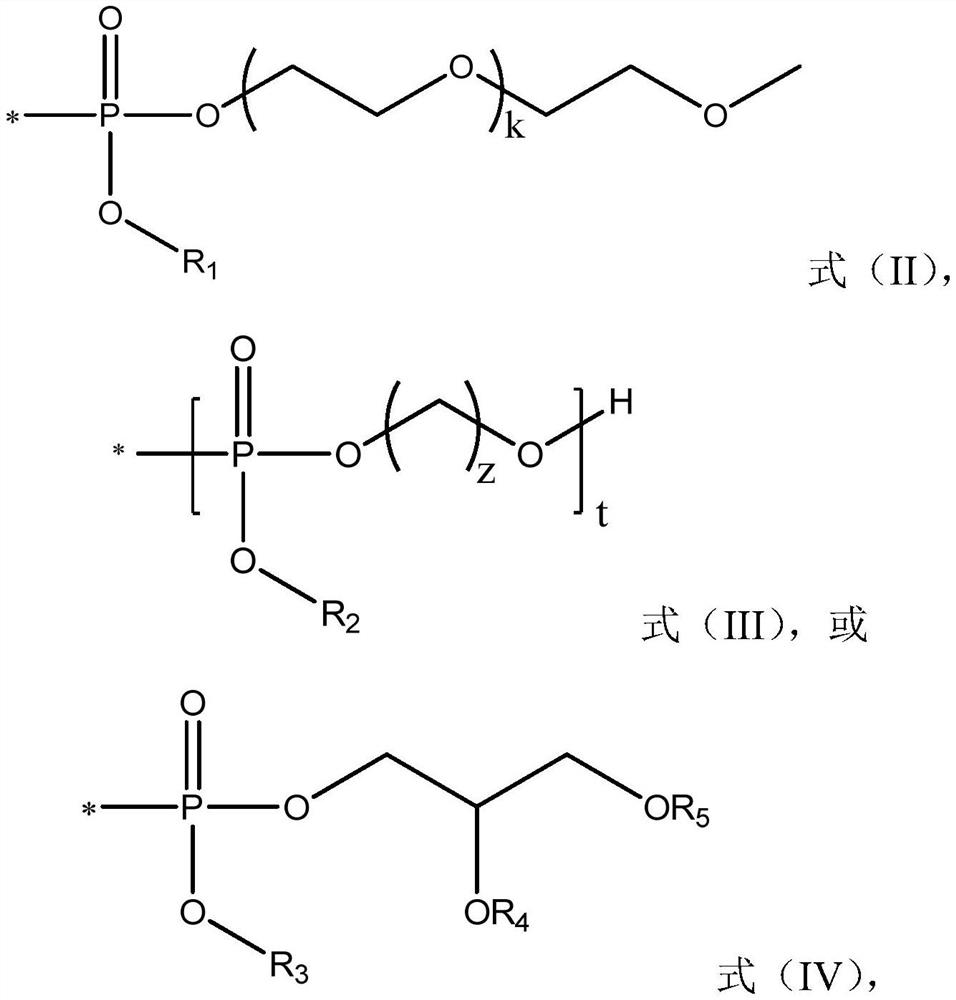
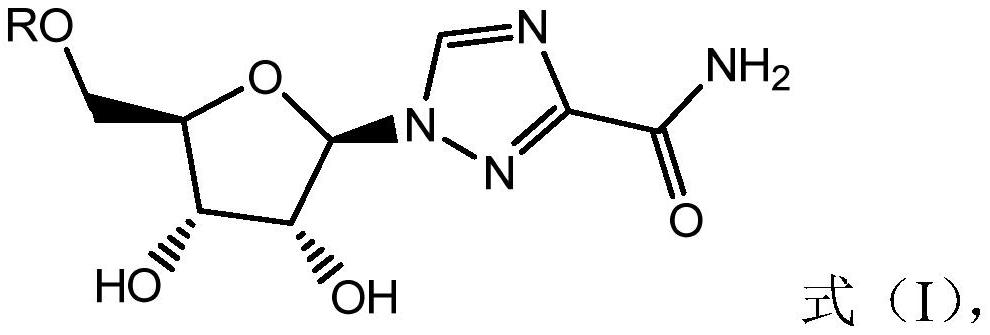
Combined use of ribavirin derivatives and alpha-interferon in the treatment and/or prevention of viral infections and related diseases caused by viral infections
A virus infection, ribavirin technology, applied in the direction of antiviral agents, medical preparations containing active ingredients, peptide/protein components, etc., can solve the problems of long course of treatment, obvious side effects, low efficiency, etc. , no side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Ribavirin derivative A1 was prepared according to the following method.
[0052]
[0053] Ribavirin derivative A1, namely compound 3, was prepared according to the process shown in formula (XII).
[0054] Purchasing dodecaethylene glycol methyl ether (compound 1, 0.5mol) according to the literature "Synthesis and Lubrication and Corrosion Research of Water-Soluble Polyethylene Glycol Phosphate" (Zhao Wei, Yuan Shuai, Yu Ping, Luo Yunbai, Lubrication Compound 2 was prepared by the method in Sealing, 2015, 40(02):64-66).
[0055] Compound 2 (0.1 mol) was treated twice with 50 mL of anhydrous pyridine and concentrated by evaporation. The residue was dissolved in 60 mL of anhydrous pyridine at room temperature, treated with 2,4,6-triisopropyl-benzenesulfonyl chloride (0.15 mol) under nitrogen, and stirred at 25°C for 4 hours. Ribavirin (0.1 mol) was then added immediately and the reaction was stirred under nitrogen for 14 hours. Hydrolysis was performed by adding 20 m...
Embodiment 2
[0057] Ribavirin derivative A2 was prepared according to the following method.
[0058]
[0059] Ribavirin derivative A2, namely compound 6, was prepared according to the process shown in formula (XIII).
[0060] Compound 5 was prepared from compound 4 by referring to the method in the literature "Synthesis of polyphosphate and its application in biomedical materials" (Hu Jian, He Jinlin, Zhang Mingzu, Ni Peihong; Polymer Bulletin, 2015(10):51-65).
[0061] Compound 5 (0.1 mol) was treated twice with 50 mL of anhydrous pyridine and concentrated by evaporation. The residue was dissolved in 60 mL of anhydrous pyridine at room temperature, treated with 2,4,6-triisopropyl-benzenesulfonyl chloride (0.15 mol) under nitrogen, and stirred at 25°C for 4 hours. Ribavirin (0.1 mol) was then added immediately and the reaction was stirred under nitrogen for 14 hours. Hydrolysis was performed by adding 20 mL of water. The solvent in the mixture was evaporated. The resulting crude mat...
Embodiment 3
[0063] Ribavirin derivative A3 was prepared according to the following method.
[0064]
[0065] Ribavirin derivative A3, namely compound 10, was prepared according to the process shown in formula (XIV).
[0066] Compound 7 was prepared by referring to the method in the literature "Synthesis of Novel Phosphate Surfactant and Its Application in Pesticide Preparation" (Pang Wenwen, Shanghai Normal University, 2014).
[0067] Compound 7 (0.8mol) was esterified with ethanol under acidic conditions, and compound 8 was purified.
[0068] Compound 8 (0.5mol) and K 2 CO 3 (5mol) was dissolved in 50mL of tetrahydrofuran, and 1-iododecane (0.5mol) was added thereto, reacted at 80°C for 12h, evaporated the solvent, purified and acidified to obtain compound 9.
[0069] Compound 9 (0.1 mol) was treated twice with 50 mL of anhydrous pyridine and concentrated by evaporation. The residue was dissolved in 60 mL of anhydrous pyridine at room temperature, treated with 2,4,6-triisopropyl-b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com