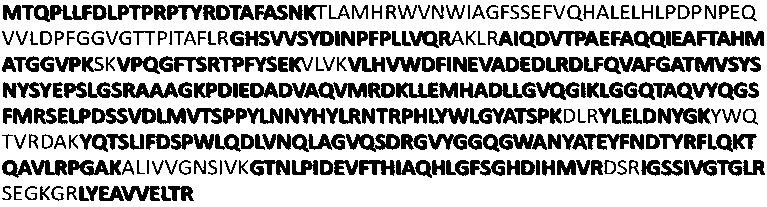DNA (deoxyribonucleic acid) methyltransferase, and soluble heterologous expression method and isolation and purification method for DNA methyltransferase
A methyltransferase, separation and purification technology, applied in the field of DNA methyltransferase and its soluble heterologous expression and separation and purification, can solve the problem of inability to obtain purified protein, in vitro expression, DNA methyltransferase expression and purification Difficulties in the process, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1: Cloning of DNA methyltransferase M.DraR1 and construction of expression vector
[0026] In order to obtain soluble target protein, the present invention optimized and screened various expression vectors (including pET-28a, pET-22b, pRRS and pET28a-HMT, etc.), hosts (including BL21, BL21(DE3), BL21(DE3) pLysS, Transetta (DE3), TransB (DE3) and E. coli ER2566, etc.) and induction expression conditions (such as IPTG concentration, induction temperature and time, etc.), and finally select soluble well-expressed pET28a-HMT and E. coli The ER2566 host was used as a prokaryotic expression system (see Table 1).
[0027] (1) Genomic DNA of Deinococcus radiodurans was extracted using the Bacterial Genomic DNA Extraction Kit (DP302-02) of Tiangen Biotechnology, and the DNA concentration and purity were determined by NANODROP 1000 (Thermo Company, USA). Design a pair of full-length specific primers for the coding gene sequence of DNA methyltransferase M.DraR1, and ...
Embodiment 2
[0036] Example 2: Prokaryotic expression of DNA methyltransferase M.DraR1
[0037] (1) Escherichia coli E. coli Preparation of ER2566 competent cells: the E. coli After the ER2566 strain (ZonHon Biopharma Institute, Inc., ZHR5015) was activated by streaking on LB solid culture dishes without any resistance, a single clone was picked and inoculated in 5 mL LB liquid medium, and cultured overnight at 37 °C with shaking at 220 rpm. ER2566 competent cells were aseptically prepared according to the competent cell preparation method in the "Molecular Cloning Guide", 100 μL was aliquoted and stored in a -80 °C ultra-low temperature refrigerator for future use.
[0038] (2) Transformation of pET28a-HMT-M.DraR1 recombinant vector: Take ER2566 competent cells from -80 ℃ ultra-low temperature refrigerator and thaw on ice, add 10-20 μg recombinant expression vector aseptically, mix lightly, and immerse in ice for 30 min , 42°C water bath heat shock for 45-90 s, then ice bath for 2-3...
Embodiment 3
[0042] Embodiment 3: Separation and purification of DNA methyltransferase M.DraR1
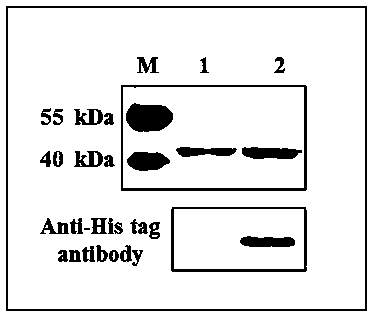
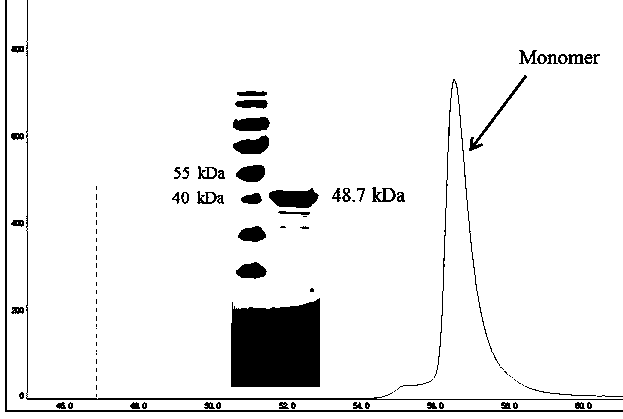
[0043] (1) Induce and cultivate the target protein according to the methods in Example 2 (3) and Example 2 (4). After the induction, collect the bacteria by centrifugation at 8000 rpm, 4 °C, 10 min, wash once with 1×PBS solution, and collect by centrifugation again Bacteria were stored at -80°C for future use. Add 20 mL lysis buffer (20 mM Tris-HCl pH8.0, 500 mM NaCl, 3 mM β-Me, 5% Glycerol, 9 mM Imidazole) to suspend cells at a ratio of 20:1. , cooled in an ice bath for 5-10 min.
[0044] (2) Cell crushing: The above suspended cells were first crushed by a high-pressure homogenizer (Shanghai Litu, FB-110 series) with parameters of 4 °C, 800-1200 bar, and 2-4 min. Continue to ultrasonically disrupt the cells in an ice-water bath (Ningbo Xinzhi, JY92-IIN), with the parameters of 60% power, 3 s ultrasonic, 9.9 s interval, and 60-90 min time. After the sonication, 4 ℃, 15000rpm, 30min high-spee...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com