Method for preparing (S)-4-decanol through biological catalysis
A technology of biocatalysis and decanol, which is applied in the direction of fermentation, etc., can solve problems such as the difficulty in obtaining chiral products with optical purity, the difficulty in achieving large-scale preparation of chiral purity, and the complex process conditions of the preparation method, achieving high yield and simple operation Ease of operation and good selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] In a 50mL glass jacketed reaction flask, add 20mL water, 0.212g KH 2 PO 4 and 0.557g K 2 HPO 4 , stir until the solid dissolves; add potassium hydroxide to adjust the pH value to 6.2-7.5; add 3g glucose, 1g ketoreductase (commercial reagent, purchased from Hequan Pharmaceutical, reagent number R308055), 0.02g NADP to the reaction solution and 0.2g GDH, after stirring, add 2g 4-decanone; adjust and control the temperature of the reaction solution to 25°C, pH 7.0, and continue to stir for 24 hours.
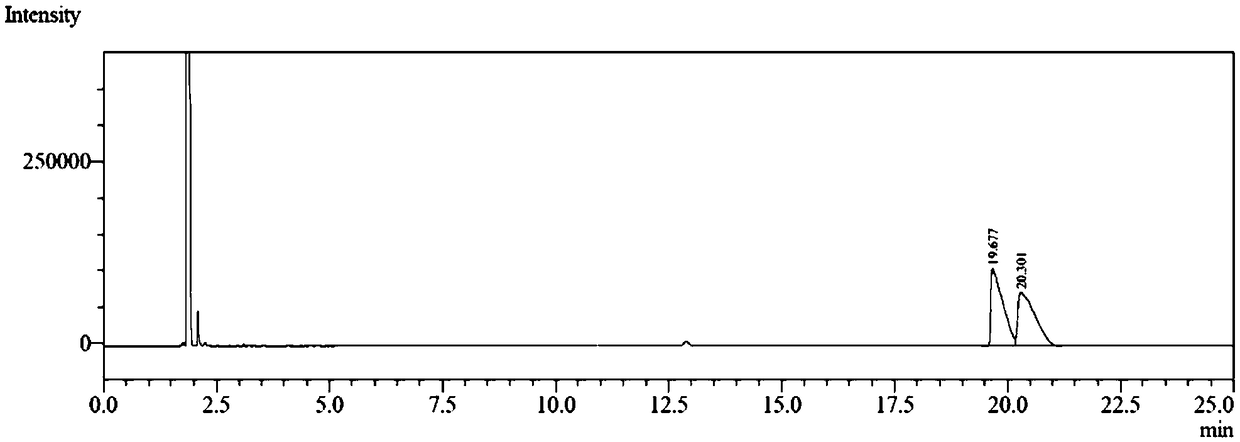
[0036]The reaction solution was extracted with 16 mL of methyl tert-butyl ether, the organic phase was collected, filtered by adding a small amount of anhydrous sodium sulfate to remove water, and concentrated by distillation under reduced pressure at 30°C. The obtained light yellow liquid is the product (S)-4-decanol, the crude yield is 92.7%, the conversion rate is 99.4% and the enantiomeric excess value is 97.6% according to the analysis by high performance gas chromato...
Embodiment 2
[0040] In a 50mL glass jacketed reaction flask, add 20mL water, 0.212g KH 2 PO 4 and 0.557g K 2 HPO 4 , stir until the solid dissolves; add potassium hydroxide to adjust the pH value to 6.2-7.5; add 2g glucose, 0.02g ketoreductase (commercial reagent, purchased from Hequan Pharmaceutical, reagent number R130709), 0.02g to the reaction solution NADP and 0.1g GDH, after stirring, add 1g of 4-decanone; adjust and control the temperature of the reaction solution to 25°C, pH 7.0, and continue to stir for 24 hours.
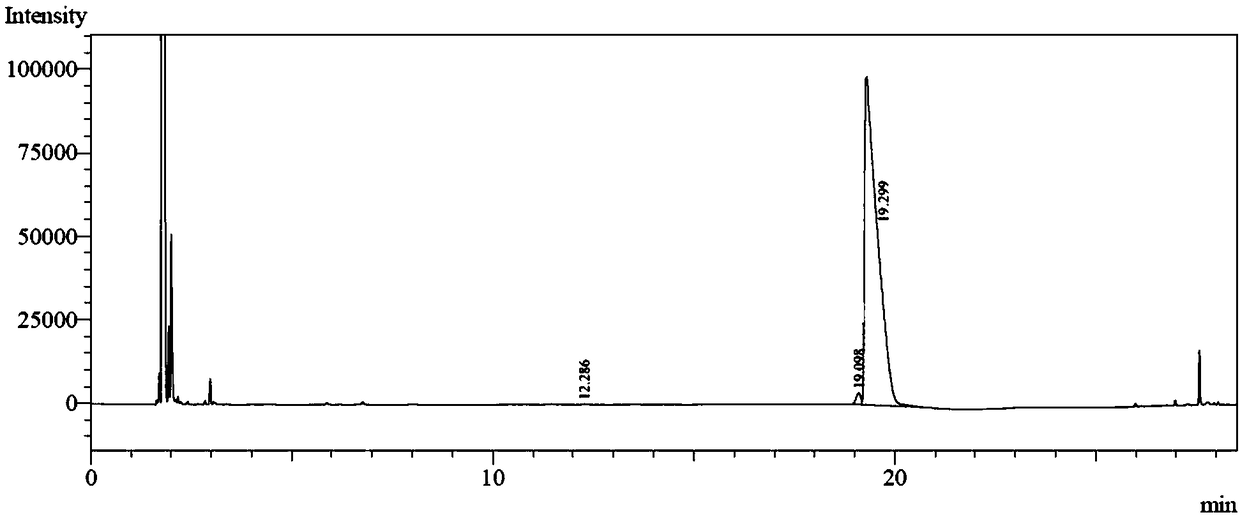
[0041] The reaction solution was extracted with 16 mL of methyl tert-butyl ether, the organic phase was collected, filtered by adding a small amount of anhydrous sodium sulfate to remove water, and concentrated by distillation under reduced pressure at 30°C. The obtained pale yellow liquid is the product (S)-4-decanol, the crude yield is 94.5%, and the conversion rate is 100% and the enantiomeric excess value is 100% through high performance gas chromatography analys...
Embodiment 3
[0044] In a 50mL glass jacketed reaction flask, add 20mL water, 0.212g KH 2 PO 4 and 0.557g K 2 HPO 4 , stir until the solid dissolves; add potassium hydroxide to adjust the pH value to 7.5; add 3g isopropanol, 1g ketoreductase (commercial reagent, purchased from Hequan Pharmaceutical, reagent number R130709) and 0.02g NAD to the reaction solution After stirring, add 2g of 4-decanone; adjust and control the temperature of the reaction solution to 25°C, pH 7.0, and continue stirring for 24 hours.
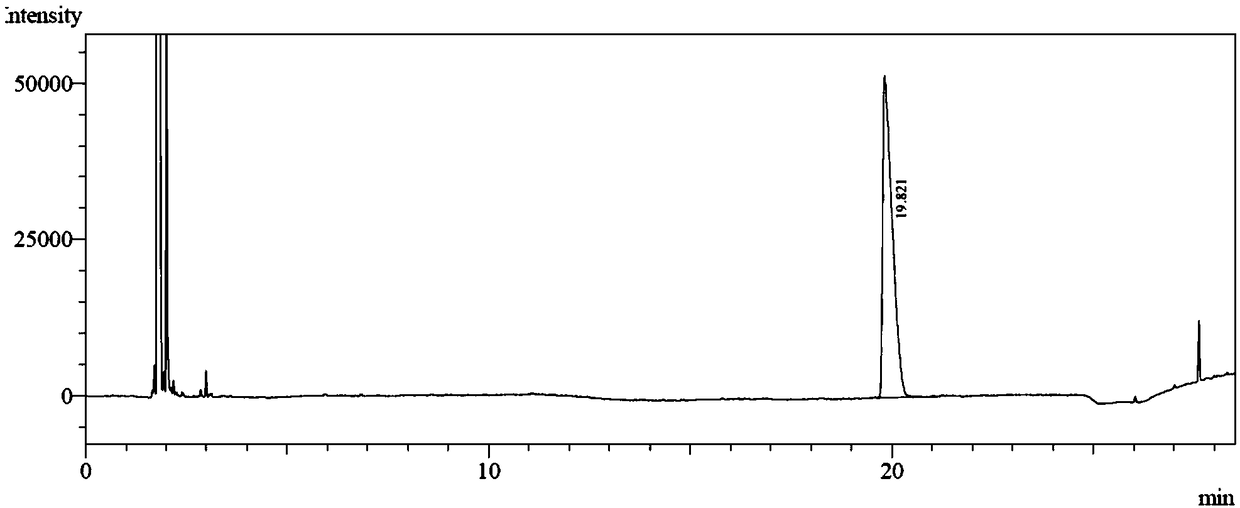
[0045] The reaction solution was extracted with 16 mL of methyl tert-butyl ether, the organic phase was collected, filtered by adding a small amount of anhydrous sodium sulfate to remove water, and concentrated by distillation under reduced pressure at 30°C. The obtained light yellow liquid is the product (S)-4-decanol, the crude yield is 96.2%, and the conversion rate is 100% and the enantiomeric excess value is 100% through high performance gas chromatography analysis. Analytic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com