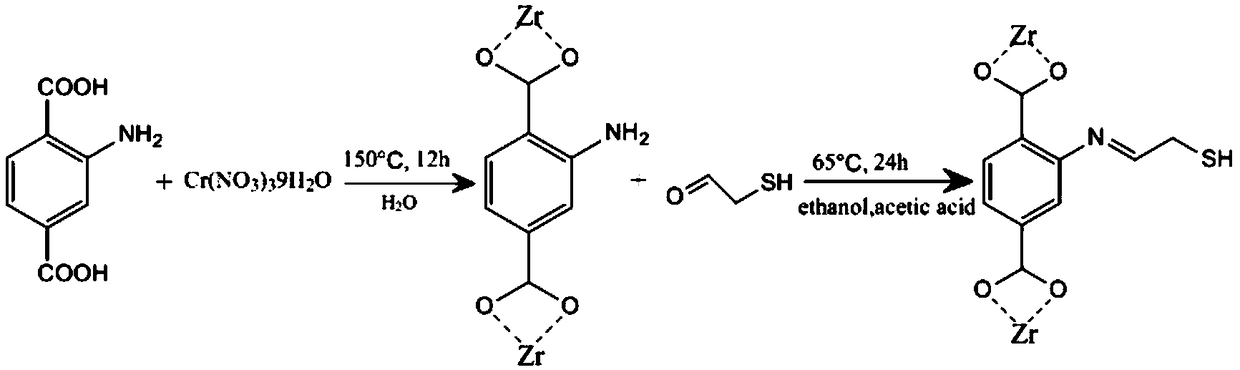
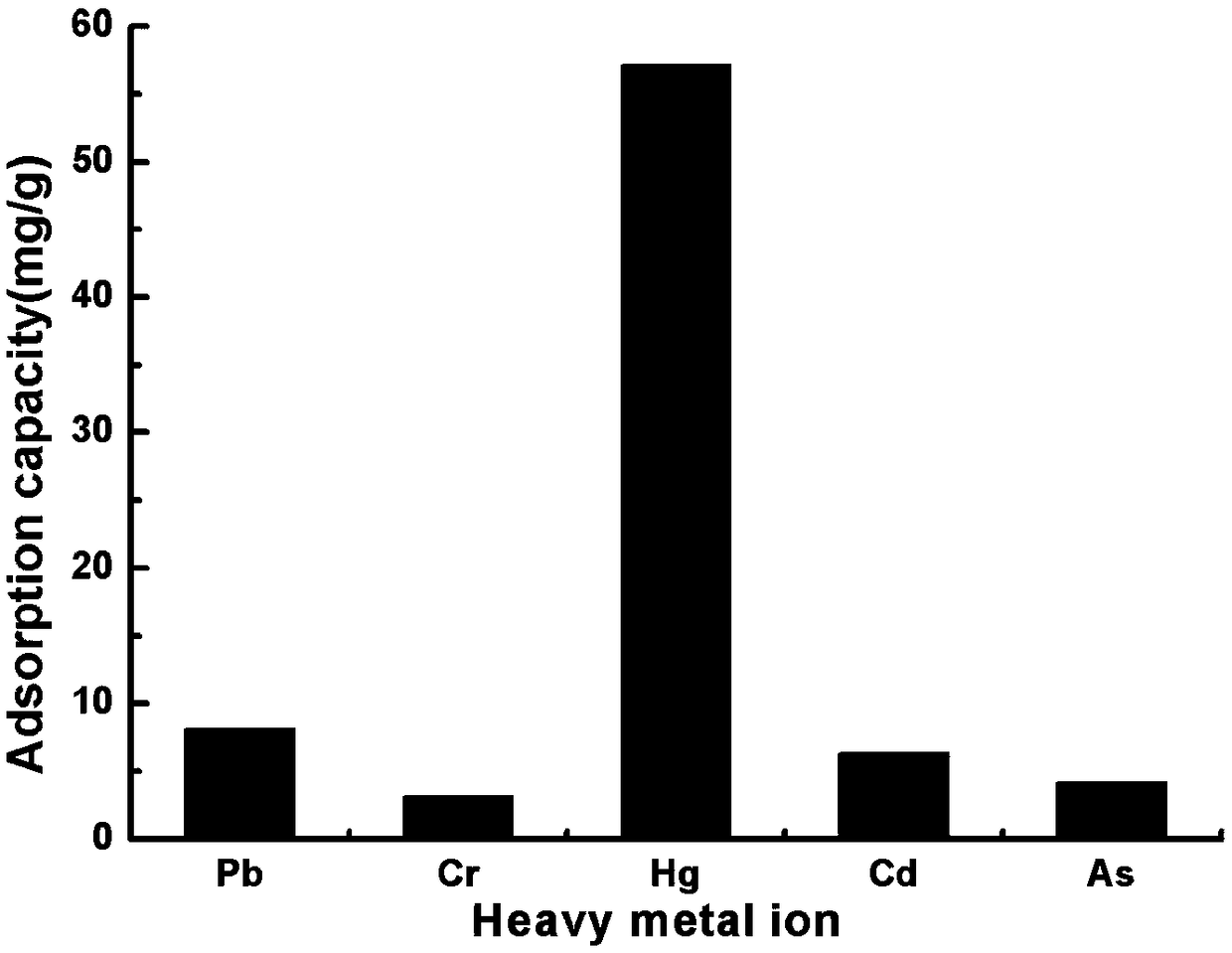
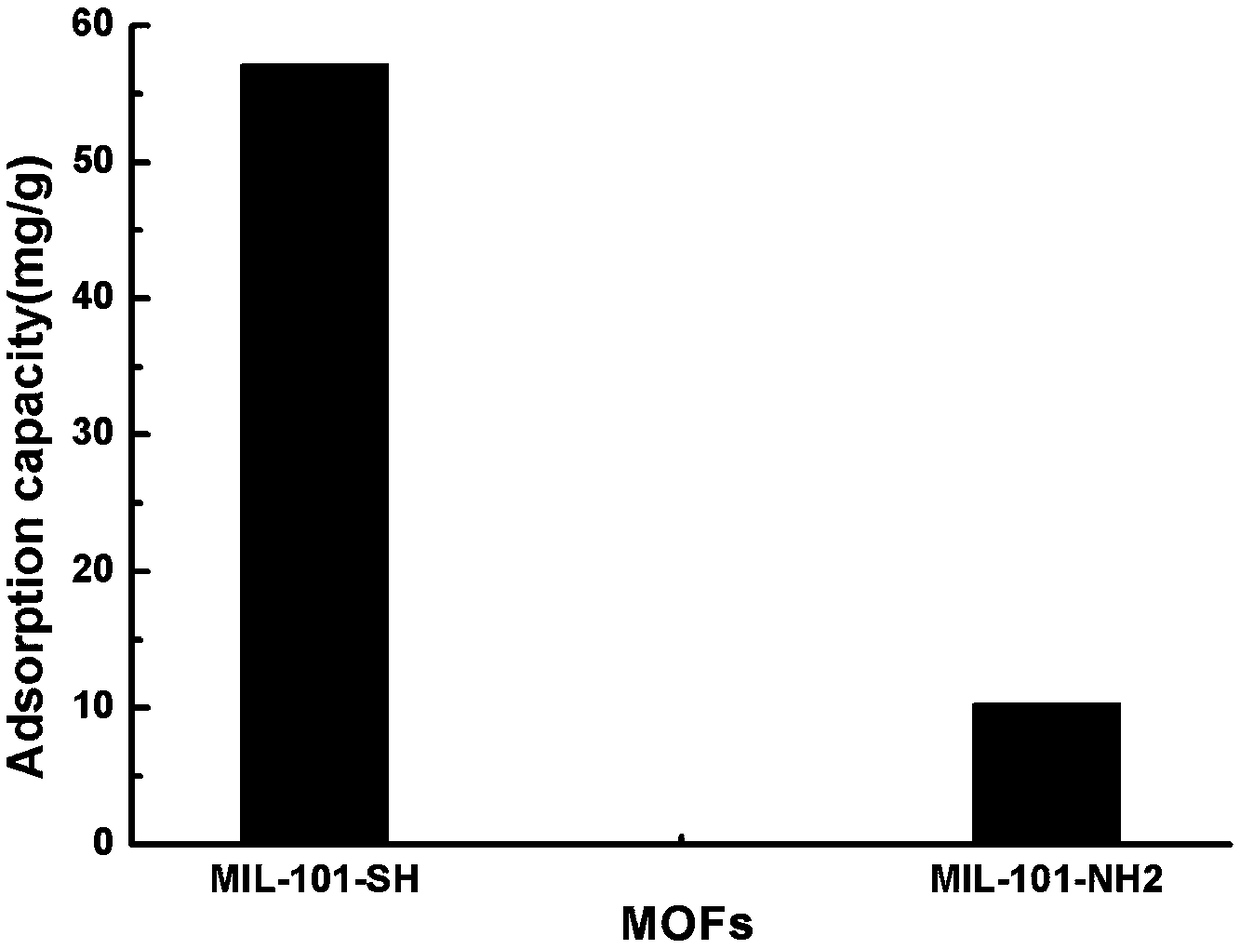
Method for synthesizing sulfydryl functionalized metal-organic framework MIL-101-SH through post-modification method
An organic framework and functionalization technology, which is used in the synthesis of thiol-functionalized metal-organic framework MIL-101-SH, can solve the problems of low adsorption capacity, limited application, and limited adsorption capacity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057]1.1. Weigh 400mg of chromium nitrate nonahydrate, 181mg of 2-aminoterephthalic acid and 100mg of sodium hydroxide, add to 7.5mL of deionized water, sonicate until completely dissolved, and transfer the mixture to a high-pressure container with a polytetrafluoroethylene liner. React in an oven at 150°C for 12 hours in a reaction kettle. After the reaction is completed, take it out of the oven, cool to room temperature and collect the solid by centrifugation, and wash the solid several times with N,N-dimethylformamide to obtain the amino functionalized metal-organic framework Cr-MIL-101-NH 2 .
[0058] 1.2. Add 19.5mL of chloroacetaldehyde to 58.8mL of deionized water at zero degrees Celsius under ice bath conditions, and adjust the pH value to about 3 with sodium carbonate solution; under ice bath conditions, completely dissolve 5.6g of sodium hydrosulfide in 33mL deionized water at zero degrees Celsius; add sodium hydrosulfide solution to chloroacetaldehyde solution, st...
Embodiment 2
[0062] 2.1. Disperse 1350.0mg of ferric chloride hexahydrate in 15mL of N,N-dimethylformamide, then dissolve 450.0mg of 2-aminoterephthalic acid in 15mL of N,N-dimethylformamide, and After the two solutions were fully mixed, they were transferred to a high-pressure reactor with a polytetrafluoroethylene liner, and reacted at 110° C. for 20 hours. After the reaction is completed, wash the solid with N,N-dimethylformamide to obtain Fe-MIL-101-NH 2 .
[0063] 2.2. Weigh 290mg Fe-MIL-101-NH 2 Mix it with 76mg of mercaptoacetaldehyde in methanol, heat and keep the temperature at 65°C in an oil bath, condense and reflux, and react for 24 hours under magnetic stirring conditions. After the reaction was completed, the solid was collected by centrifugation, and the solid was washed several times with methanol to obtain the mercapto functionalized metal-organic framework Fe-MIL-101-SH.
Embodiment 3
[0065] 3.1. Dissolve 272mg of 2-aminoterephthalic acid in 60mL of N,N-dimethylformamide and heat to 110°C in an oil bath. Divide 724mg of aluminum trichloride hexahydrate into seven equal portions and add to the above solution, with an interval of 15 minutes between two times. Continue magnetic stirring for 3 hours after the last addition, and continue to react at 110°C for 16 hours after the stirring is stopped. After the reaction is completed, wash the solid with N,N-dimethylformamide and ethanol to obtain Al-MIL-101- NH 2 .
[0066] 3.2. Weigh 230mg Al-MIL-101-NH 2 Mix it with 76mg of mercaptoacetaldehyde in methanol, heat and keep the temperature at 65°C in an oil bath, condense and reflux, and react for 24 hours under magnetic stirring conditions. After the reaction was completed, the solid was collected by centrifugation, and the solid was washed several times with methanol to obtain the mercapto functionalized metal-organic framework Al-MIL-101-SH.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com