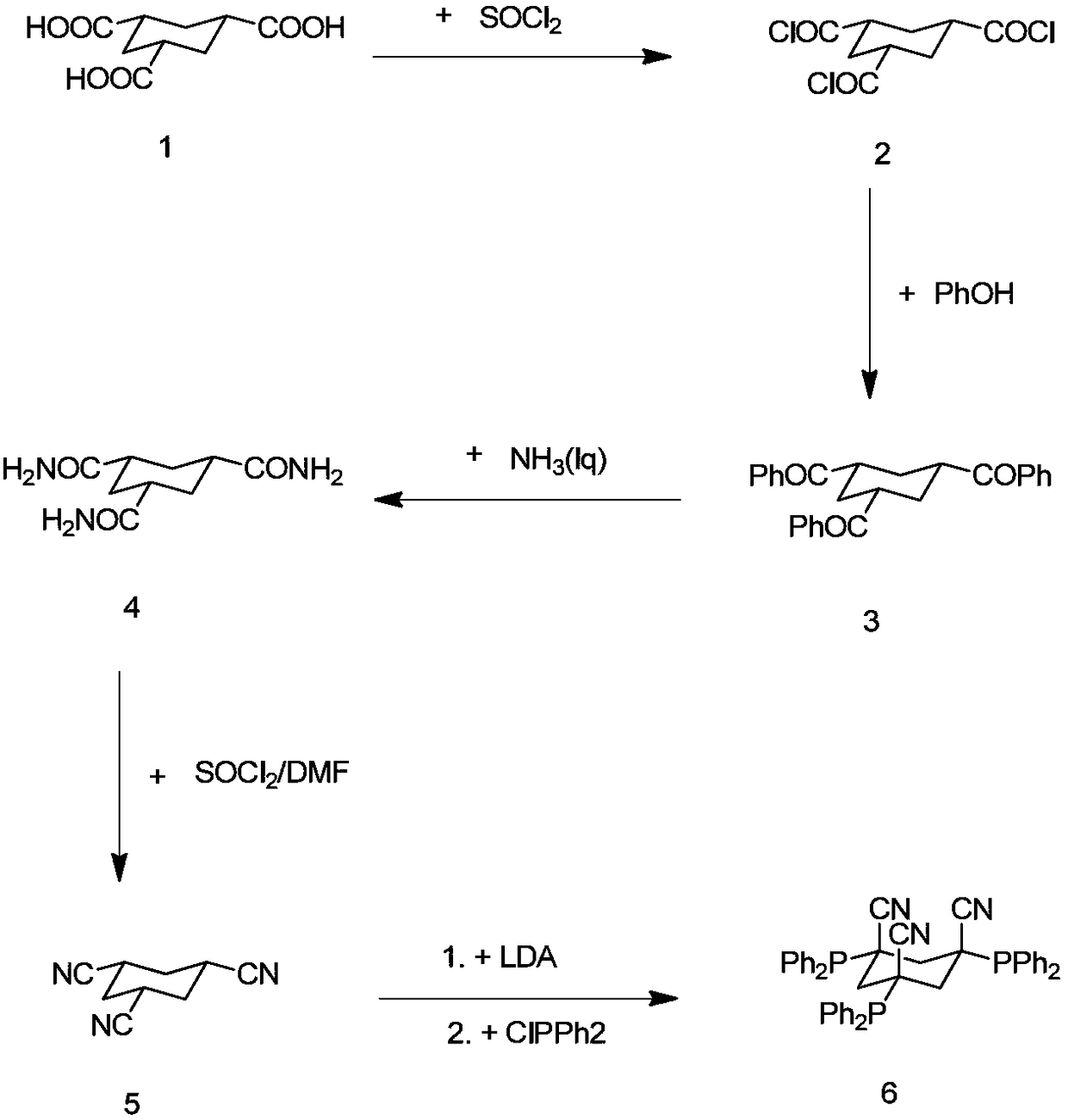
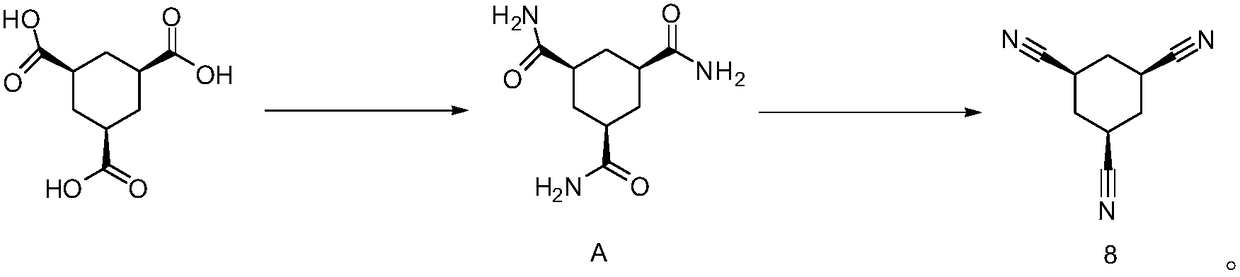
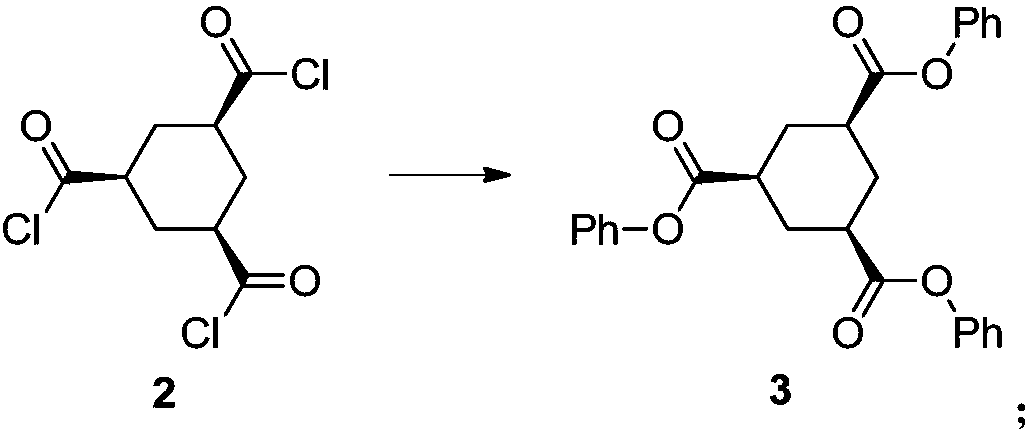
Multi-step continuous preparation method of (1alpha, 3alpha and 5alpha)-1,3,5-cyclohexane tricarbonitrile
A technology of cyclohexanetriamide and triethylamine, which is applied in the field of preparation of -1,3,5-cyclohexanetrinitrile, can solve the problems of many wastes, complicated operation and low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] (1) Add the reaction solvent 1,2-dichloroethane (4.0eq, calculated based on 1.0eq of cis trimesic acid, the equivalents in the examples are the same) into the dry reaction flask, and then add 20.0g of raw materials cis trimesic acid (compound 1, 1.0eq) and 0.3g catalyst DMF (0.05eq), magnetically stirred. The internal temperature of the system was heated to 65 degrees, and thionyl chloride (3.3 eq) was added dropwise, and the dropping temperature was controlled at 65-70 degrees. After the dropwise addition, keep the temperature at 65-70°C for 3 hours. After the reaction is complete, steam at 75-85°C under normal pressure to collect 1,2-dichloroethane (2eq, steam out half of the amount of 1,2-dichloro Ethane is used for subsequent batches, and the system is cooled to room temperature for the next reaction.
[0076] (2) The reaction solution of step (1) is cooled to 20 degrees of internal temperature, and the mixed solution of triethylamine (3.6eq) and phenol (3.3eq) i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com