A method for recycling residual lithium in the reaction mother liquor of electrode material prepared by hydrothermal method
An electrode material and recycling technology, applied in the direction of battery electrodes, circuits, electrical components, etc., can solve the problems that lithium sulfate cannot be recycled, the recycling scheme is cost-effective, and the scope of application is narrow, which is conducive to large-scale promotion and application, recycling Good recycling effect and wide application range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Take the hydrothermal method to prepare the reaction mother liquor of lithium iron phosphate, raise the temperature to 80°C, add lithium hydroxide, adjust the pH value to greater than 11, keep it for 2 hours, and form lithium phosphate and iron hydroxide precipitate suspension; centrifuge to separate the solid and liquid to obtain solid precipitate respectively substances and clear solutions.
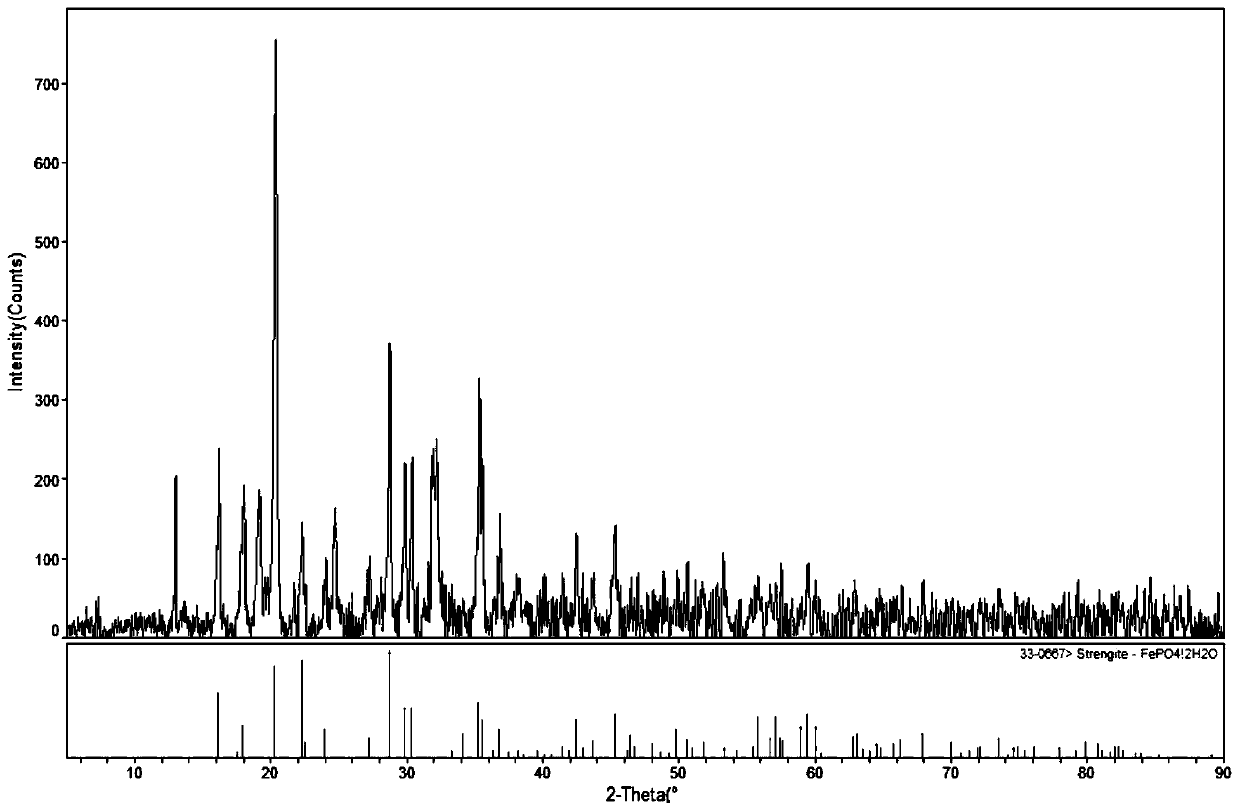
[0035] Add the solid precipitate to phosphoric acid at a temperature of 60°C, add 60 mL of water to 5 g of the precipitate, and add phosphoric acid to 1.05 times the theoretical molar value of the reaction with the precipitate. Insulated for 2 hours to obtain a white iron phosphate precipitate, its XRD pattern is shown in figure 1 , Centrifugal separation to obtain a mixed clear solution of lithium dihydrogen phosphate and phosphoric acid, no other chemical raw materials are added, no need to remove impurities, high purity, can be used as raw material for the production of lithiu...
Embodiment 2
[0039] Take the mother liquor of lithium manganese phosphate, raise the temperature to 60°C, add lithium hydroxide, adjust the pH value to be greater than 11, and keep it warm for 3 hours to form lithium phosphate and manganese hydroxide precipitated suspension; centrifuge to separate solid and liquid to obtain solid precipitate and clarified solution respectively.
[0040] Add the solid precipitate to the phosphoric acid solution at a temperature of 40°C, add 60 mL of water to 5 g of the precipitate, and add phosphoric acid to 1.05 times the theoretical molar value of the precipitate. After heat preservation for 10 hours, a white manganese phosphate precipitate was obtained, and centrifuged to obtain a mixed clear solution of lithium dihydrogen phosphate and phosphoric acid, which can be used as a raw material for producing lithium manganese phosphate by the hydrothermal method. The recovery of lithium in the precipitate was >95%.
[0041] Put the clarified solution into a homo...
Embodiment 3
[0044] Take the mother liquor of lithium manganese iron phosphate, raise the temperature to 100°C, add lithium hydroxide, adjust the pH value to be greater than 10, and keep it warm for 1 hour to form lithium phosphate, manganese hydroxide and iron hydroxide precipitate suspension; centrifuge to separate the solid and liquid to obtain solid precipitate respectively substances and clear solutions.
[0045] Add the solid precipitate to phosphoric acid at a temperature of 80°C, add 60 mL of water to 5 g of the precipitate, and add phosphoric acid to 1.05 times the theoretical molar value of the reaction with the precipitate. After 1.5 hours of heat preservation reaction, white manganese phosphate and iron phosphate precipitates were obtained, and centrifuged to obtain a mixed clear solution of lithium dihydrogen phosphate and phosphoric acid, which can be used as a raw material for producing lithium manganese iron phosphate by the hydrothermal method. The recovery of lithium in t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
| rate of recovery | aaaaa | aaaaa |
| rate of recovery | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com