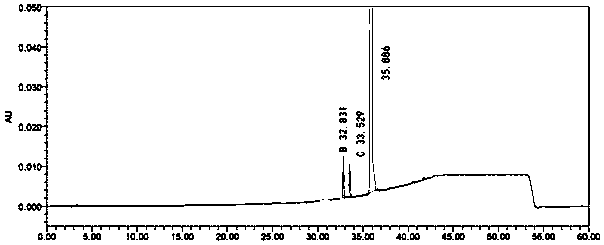
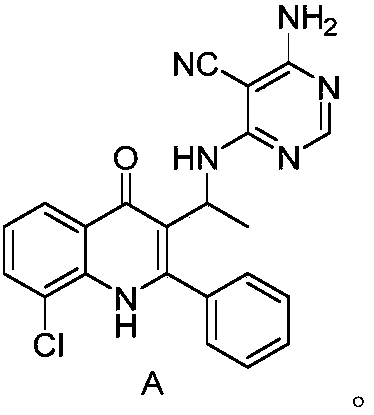
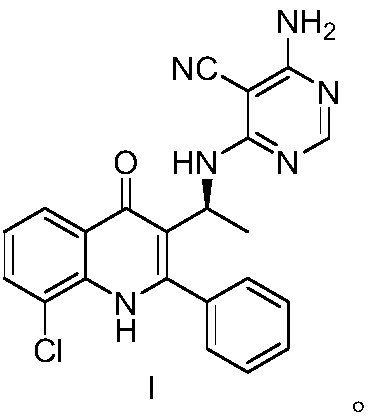
Substituted pyrimidine type compound as well as preparation method and use thereof
A compound, pyrimidine technology, applied in the field of medicinal chemistry, can solve problems such as druggability, safety and efficacy hidden dangers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1 (S)-4-amino-6-((1-(8-chloro-4-oxo-2-phenyl-1,4-dihydroquinolin-3-yl)ethyl)amino) Preparation of pyrimidine-5-cyanomethanesulfonate
[0035] Step 1: Preparation of 3-chloroisatoic anhydride
[0036]
[0037] Add 2-amino-3-chlorobenzoic acid (34.2g, 0.2mol) and 175mL of acetonitrile in a 500mL three-necked flask, cool down to 0°C, then add dropwise a dichloromethane solution of triphosgene (triphosgene (29.6g, 0.1 mol) was dissolved in 150 mL of dichloromethane), and pyridine (50 mL, 0.6 mol) was added dropwise at the same time, and the dropwise addition was completed in about 30 minutes, and the reaction was tracked by TLC until the reaction was complete. After treatment, spin dry directly, add water to make a slurry, and filter to obtain the title compound.
[0038] Step 2: Preparation of 3-acetyl-8-chloro-2-phenylquinolin-4(1H)-one
[0039]
[0040] Add 1-phenyl-1,3-butanedione to the reaction flask, add N,N-dimethylformamide, add sodium methoxide to...
Embodiment 2
[0059] Example 2: (S)-4-amino-6-((1-(8-chloro-4-oxo-2-phenyl-1,4-dihydroquinolin-3-yl)ethyl)amino ) Preparation of pyrimidine-5-methylamide
[0060]
[0061] 5g of (S)-4-amino-6-((1-(8-chloro-4-oxo-2-phenyl-1,4-dihydroquinolin-3-yl)ethyl)amino) Pyrimidine-5-cyano was dissolved in a mixed solvent of 10mL methanol / 40mL water, refluxed at 100°C for 12 hours, turned off the heating and returned to room temperature, concentrated to dryness, and the crude product was obtained by sand-making and rapid column flushing, and the white pure title compound. ESI-MS m / z:457.2[M+Na] + . 1 H NMR (500MHz, DMSO-d 6 )δ: 1.39(3H,J=6.8Hz,d),5.17-5.11(1H,m),6.46(2H,s),7.34(2H,s),7.38-7.36(1H,m),7.56-7.58 (5H,m),7.75(1H,s),7.83(1H,J=7.5Hz,d),8.09(1H,J=8.5Hz,d),8.16(1H,J=8.0Hz,d),10.75 (1H,s).
Embodiment 3
[0062] Example 3: (S)-6-amino-4-(1-(8-chloro-4-oxo-2-phenyl-1,4-dihydroquinolin-3-yl)ethylamino)-5 - Preparation of cyanopyrimidine-1-nitrogen oxide
[0063]
[0064] 4g (S)-4-amino-6-((1-(8-chloro-4-oxo-2-phenyl-1,4-dihydroquinolin-3-yl) ethyl) amino) pyrimidine -5-cyano was dissolved in toluene, 8.3 g of m-chloroperoxybenzoic acid was added, heated and stirred at 80°C for 4 hours, filtered, the mother liquor was concentrated to obtain a yellow solid, and the title compound was obtained by preparative liquid phase separation. ESI-MS m / z:455.2[M+Na] + . 1 H NMR (500MHz, DMSO-d 6 )δ: 1.39(3H,J=6.5Hz,d),5.05(1H,m),7.41(1H,J 1 =J 2 =8.0Hz,dd),7.54-7.52(2H,m),7.59-7.55(3H,m),7.88(2H,J 1 =7.5Hz,J 2 =1.0Hz,dd),8.17(1H,J=9.0Hz,d),8.23(1H,J 1 =7.5Hz,J 2 =1.0Hz,dd),8.35(1H,s),11.10(1H,s).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com