Polypeptide, its preparation method and use for inhibiting HIV
A technology of use and carrier, applied in the field of therapeutic agents for inactivating HIV, can solve the problems of inability to clear HIV latent reservoirs to cure AIDS, inability to kill HIV-infected cells, etc., and achieve the effect of resistant strains
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
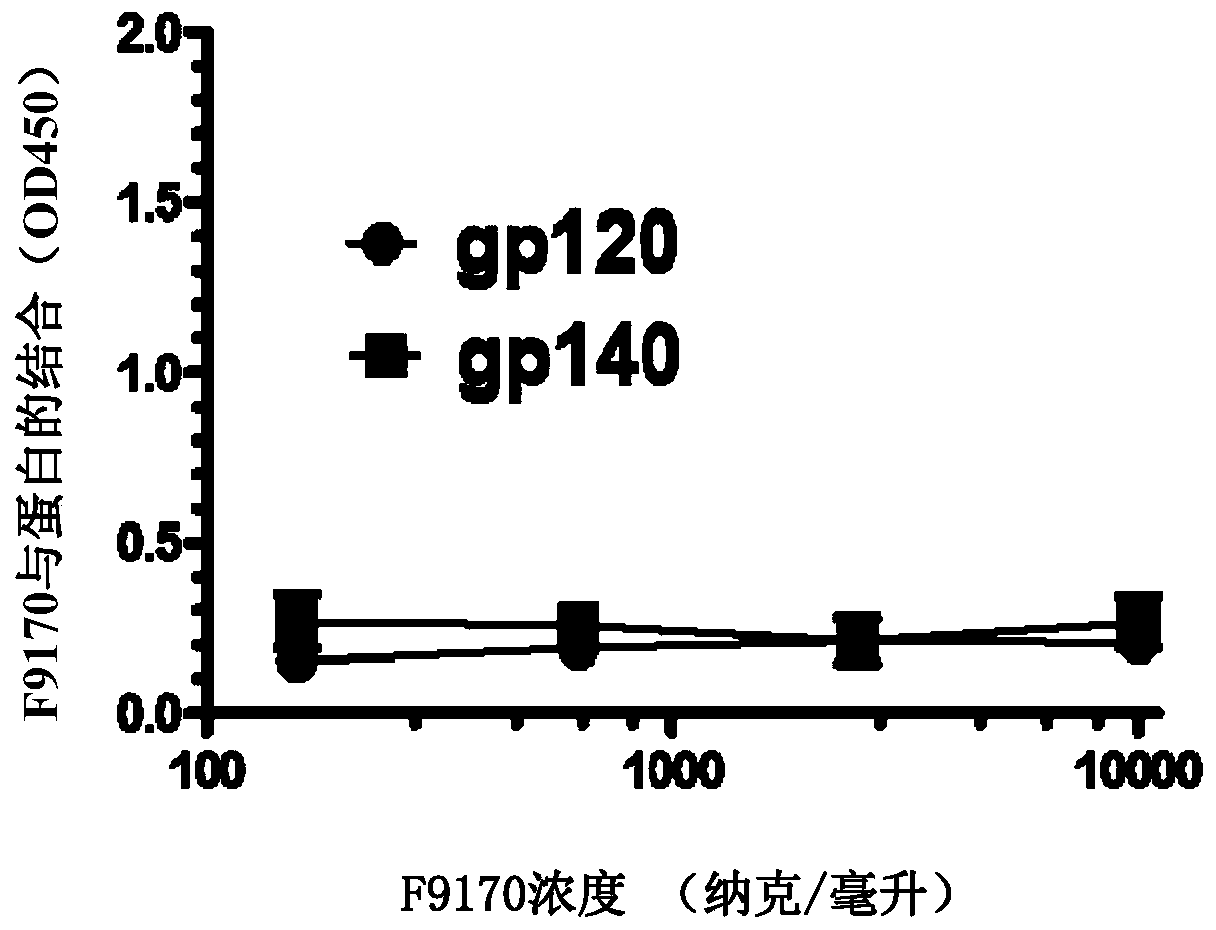
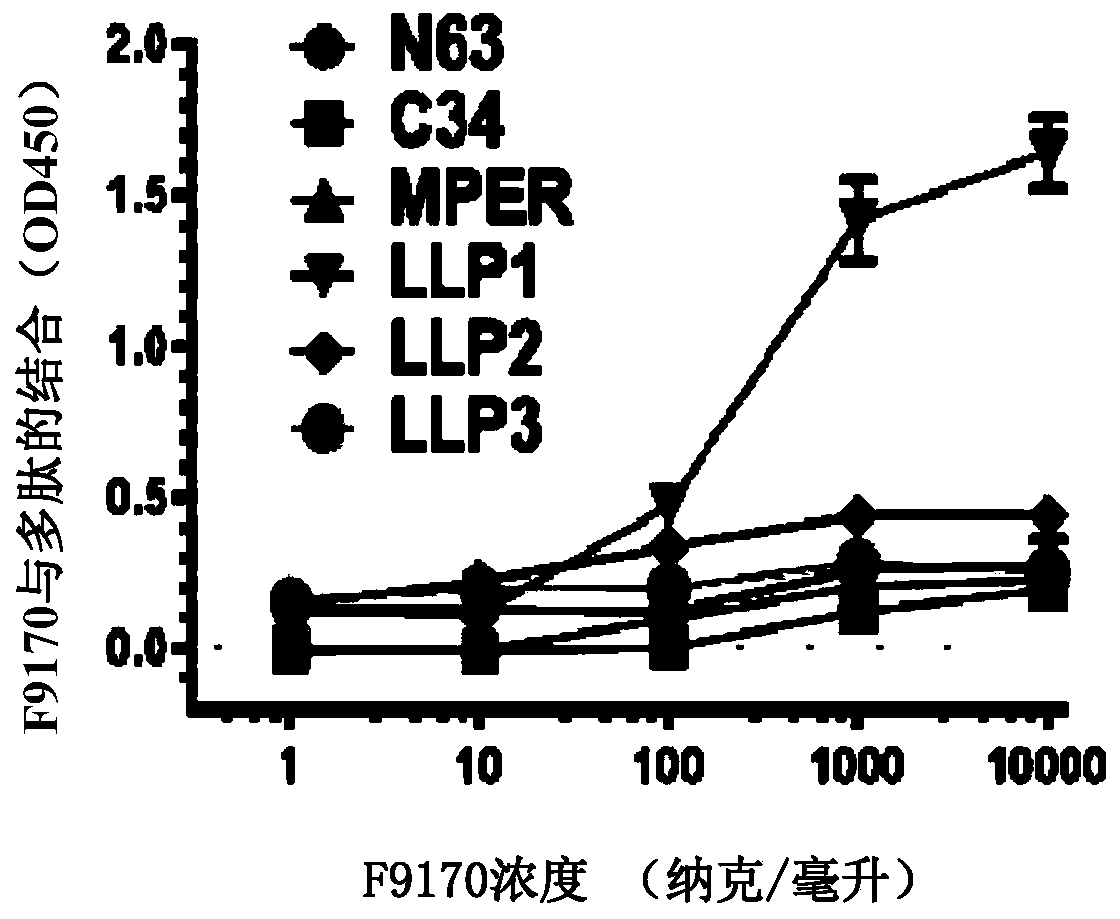
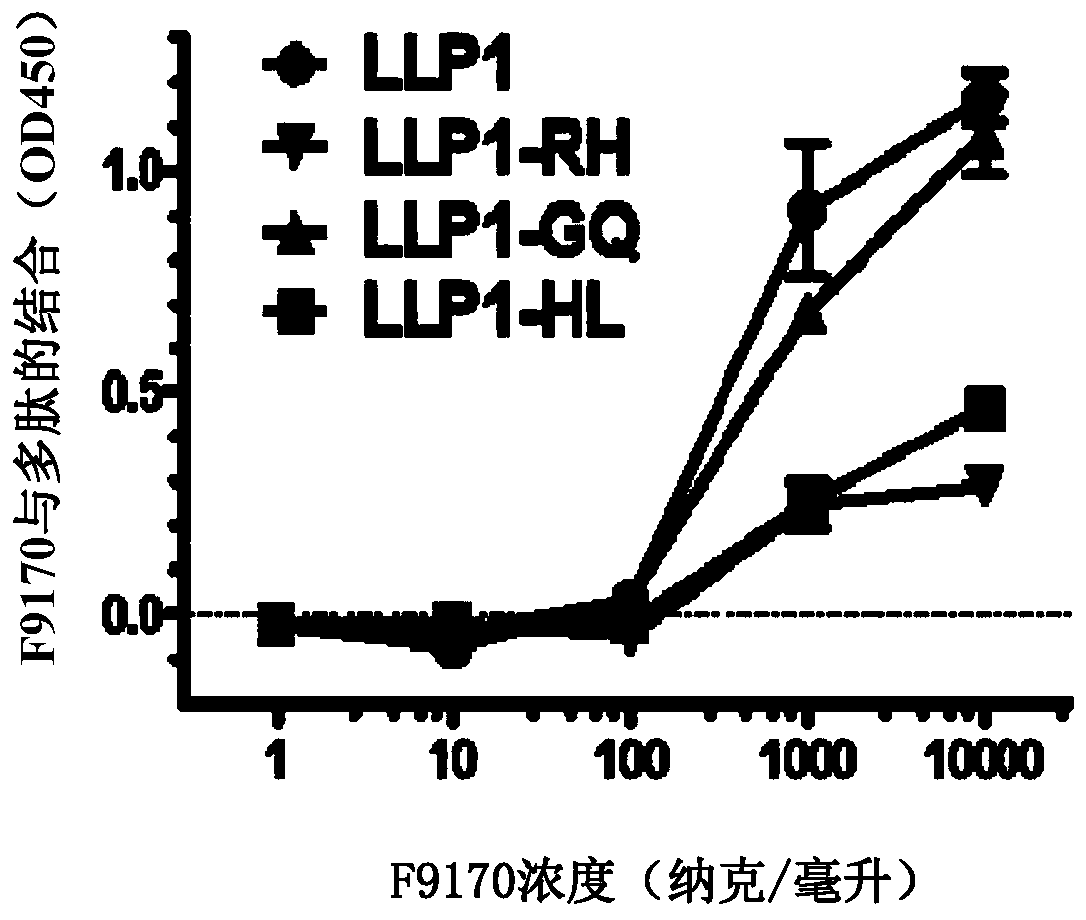
[0096] Embodiment 1: Binding experiment of the polypeptide of the present invention
[0097] 1.1 Experimental materials and methods
[0098]Based on enzyme-linked immunosorbent assay (ELISA), it was detected whether the F9170 polypeptide (SEQ ID NO: 2) (synthesized by Synpeptide Co., Ltd.) could interact with the following HIV envelope protein-derived proteins or polypeptides (all Synpeptide Co., Ltd. Synthetic) combination: gp120 (SEQ ID NO:19): HIV envelope protein gp120 subunit; gp140 (SEQ ID NO:20): HIV envelope protein removes the transmembrane domain and intracellular sequence part; N63 (SEQ ID NO:21): NHR domain on HIV envelope protein subunit gp41; C34 (SEQ ID NO:22): CHR domain on HIV envelope protein subunit gp41; MPER (SEQ ID NO:23): HIV envelope Proximal membrane domain on protein subunit gp41; LLP1(SEQ ID NO:24) / LLP2(SEQ ID NO:25) / LLP3(SEQ ID NO:26): HIV envelope protein subunit gp41 intracellular segment LLP1 / LLP2 / LLP3 domain; LLP1-RH (SEQ ID NO: 27): 828-842 ...
Embodiment 2
[0102] Embodiment 2: Polypeptide of the present invention inactivates HIV virus
[0103] 2.1 Experimental materials and methods
[0104] Detection of F9170 polypeptide (SEQ ID NO:2), F9170 scrambled polypeptide (F9-scr) (SEQ ID NO:30) and T20 (SEQ ID NO:18) (all synthesized by Synpeptide Co., Ltd.) to inactivate HIV Virus strain HIV-1IIIB, Bal, TZA68 / 125A and 92TH009 virus strain (from NIH AIDS Reagent Program, product number is 398,510,11255,1656 respectively) The method of ability is as follows: each 50 μ L above-mentioned each HIV virus (with serum-free 1640 medium (Meilunbio, product number MA0215, the 1640 medium used in all embodiments is this product) dilution, the final concentration is 200 times TCID 50 ) and 50 μL diluted (4 times) F9170 polypeptide, F9170 scrambled polypeptide and T20 (diluted with serum-free 1640 medium, initial concentration is 5 μM) and incubated at 4°C for 1 hour. Add PEG6000 (Sigma-Aldrich, Cat. No. 1546580) to make the final concentration 3%...
Embodiment 3
[0108] Example 3. F9170 specifically inhibits HIV pseudoviruses.
[0109] 3.1 Experimental materials and methods
[0110] Pseudovirus packaging: HIV envelope protein particles or MERS-CoV envelope protein particles (HIV envelope protein particles are from AIDS Reagent Program, item number is 324; MERS-CoV envelope protein particles are from cooperative research group New York Blood Center Viral Immunology research group) and HIV backbone plasmid (pNL4-3.Luc.R-E, from NIH AIDS ReagentProgram, item number: 3418) were used to package HIV and MERS-CoV pseudoviruses. Two kinds of plasmid HIV envelope protein particles or MERS-CoV envelope protein particles and HIV backbone plasmid (5 μg DNA melted in 100 μl normal saline) were simultaneously used VigoFect reagent (Vigolas Biotechnology (Beijing) Co., Ltd., catalog number T001) ( 2 μl of reagent diluted to 100 μl of saline) were transfected into 293T cells (purchased from ATCC) according to the manufacturer's instructions (200,000-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com