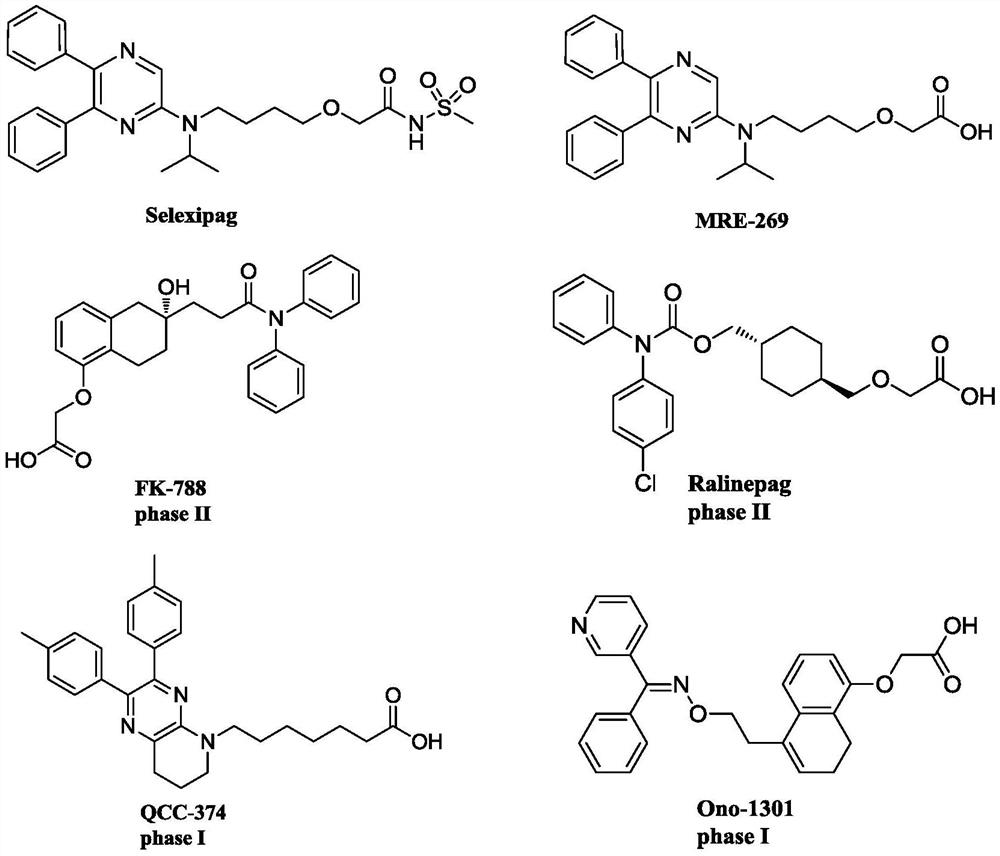
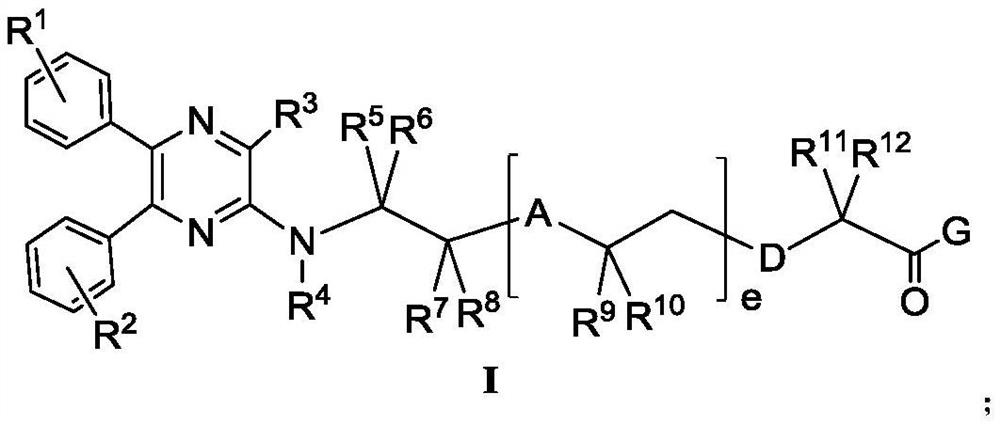
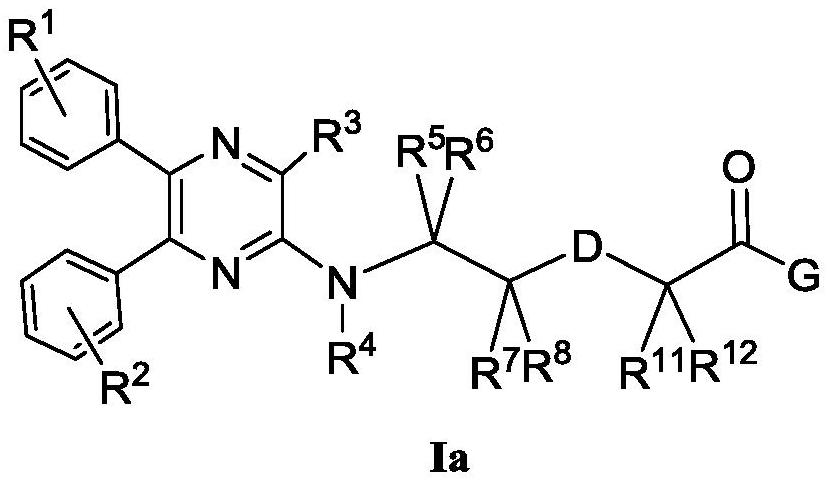
Aminopyrazine compound or salt, isomer, preparation method and use thereof
A technology of aminopyrazines and compounds, applied in the field of medicinal chemistry, can solve the problems of reducing anti-platelet aggregation activity and achieve the effects of improving anti-platelet aggregation activity, reducing mean pulmonary hypertension, and improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0183] Example 1 Preparation of 2-{2-[N-(5,6-diphenylpyrazin-2-yl)-N-isopropylamino]ethoxy}acetic acid
[0184]
[0185] Step 1: Preparation of 5,6-diphenyl-2-hydroxypyrazine
[0186]
[0187] Under nitrogen protection, bibenzoyl (31.0g, 0.10mol), aminoacetamide (13.0g, 0.12mol) and NaOH (7.00g, 0.24mol) were sequentially added to 1L methanol solvent, heated to reflux for 3-4h , the reaction was monitored by LC-MS, and the starting material was completely reacted. Cool the reaction solution to 0-5°C, then add 12.5mL 12N HCl solution dropwise, and stir the reaction solution at room temperature for 30min, then add 10g sodium bicarbonate and 130mL water, filter the reaction solution, wash the solid with a small amount of water and methanol respectively , dried in vacuo to obtain 22.0 g of white solid 5,6-diphenyl-2-hydroxypyrazine, yield: 88.7%, ESI-MS: m / z=249.2 (M+H) + .
[0188] Step 2: Preparation of 5-chloro-2,3-diphenylpyrazine
[0189]
[0190] Under the prote...
Embodiment 2
[0200] Example 2 Preparation of 2-{2-[N-(5,6-diphenylpyrazin-2-yl)-N-methylamino]ethoxy}acetic acid
[0201]
[0202] The synthesis method is the same as in Example 1, the only difference being that 2-(N-isopropylamino)ethanol in step 3 of Example 1 is replaced by N-methylaminoethanol to obtain yellow oily 2-{2- [N-(5,6-diphenylpyrazin-2-yl)-N-methylamino]ethoxy}acetic acid; ESI-MS: m / z=364.2(M+H) + ; 1 H NMR (400MHz, d 6 -DMSO)δ12.64(s,1H),8.19(s,1H),7.37-7.20(m,10H),4.07(s,2H),3.71-3.62(m,4H),3.13(s,3H) .
Embodiment 3
[0203] Example 3 Preparation of 2-{2-[2-(N-(5,6-diphenylpyrazin-2-yl)-N-isopropylamino)ethoxy]ethoxy}acetic acid
[0204]
[0205] Step 1: Preparation of 5,6-diphenyl-2-hydroxypyrazine
[0206]
[0207] Under the protection of nitrogen, add bibenzoyl (63.0g, 0.30mol), aminoacetamide (39.0g, 0.36mol) and NaOH (29.0g, 0.72mol) to 1L methanol solvent in sequence, and heat to reflux for 3-4h , the reaction was monitored by LC-MS, and the starting material was completely reacted. Cool the reaction solution to 0-5°C, then add 37.5mL 12N HCl solution dropwise, and stir the reaction solution at room temperature for 30min, then add 30g sodium bicarbonate and 380mL water, filter the reaction solution, wash the solid with a small amount of water and methanol respectively , dried in vacuo to obtain 68.0 g of white solid 5,6-diphenyl-2-hydroxypyrazine, yield: 91.4%, ESI-MS: m / z=249.2 (M+H) + .
[0208] Step 2: Preparation of 5-chloro-2,3-diphenylpyrazine
[0209]
[0210] Under ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com