Hematopoietic stem cell culture medium
A technology of hematopoietic stem cells and culture medium, applied in the field of cell culture, can solve problems such as poor activity and proliferation ability, and achieve the effect of protecting growth factor activity, continuous release and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
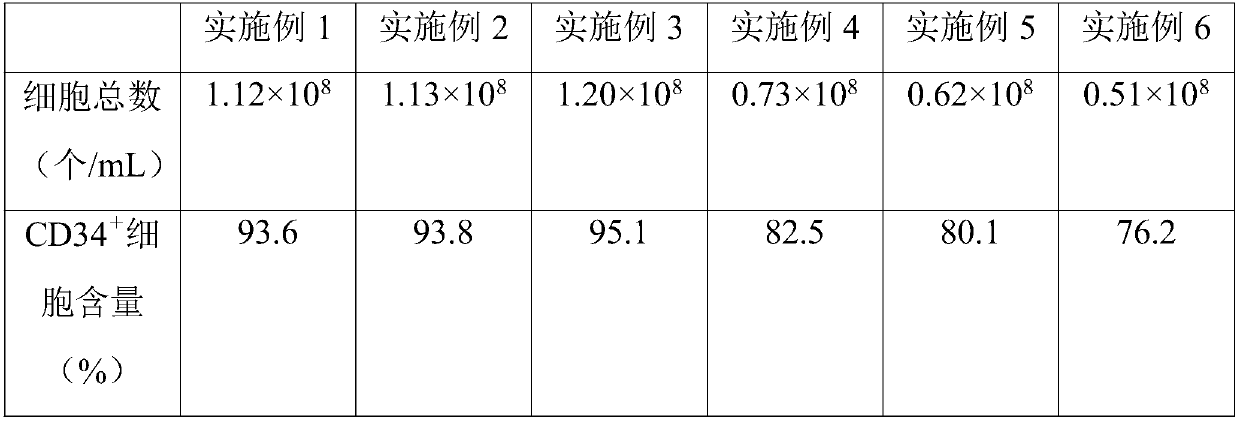
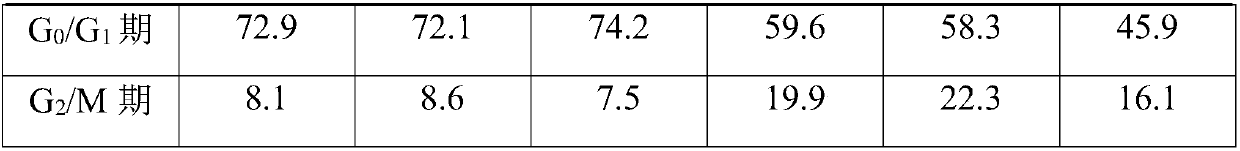
[0036]A kind of hematopoietic stem cell culture medium, comprises basal medium and additive, and basal medium is DMEM culture medium (Beijing Qingda Tianyi Science and Technology Co., Ltd.), and by final concentration, additive comprises the component of following content: trehalose 60ng / mL , Vitamin C 50ng / mL, Berberine 1mg / mL, Stephanine 30μmol / mL, the first cytokine sustained-release sphere 1.2mg / mL, the second cytokine sustained-release sphere 15mg / mL, sodium chloride 3mg / mL , glutathione 20mg / mL, hyaluronic acid 5μg / mL, grape seed extract 2mg / mL, ginsenoside 6mg / mL and tremella polysaccharide 5mg / mL.
[0037] Wherein, the first cytokine sustained-release sphere is prepared by the following method:
[0038] (1) adding dextran and polyethylene glycol into water respectively, and stirring until dissolved to obtain 8wt% dextran aqueous solution and 8wt% polyethylene glycol aqueous solution;
[0039] (2) At 0-4°C, mix dextran aqueous solution and polyethylene glycol aqueous s...
Embodiment 2
[0053] A hematopoietic stem cell culture medium, including basal medium and additives, the basal medium is DMEM medium, and the additives include the following components in terms of final concentration: trehalose 80ng / mL, vitamin C65ng / mL, berberine 3mg / mL, Stephanine 50μmol / mL, the first cytokine sustained-release sphere 2.5mg / mL, the second cytokine sustained-release sphere 30mg / mL, sodium chloride 8mg / mL, glutathione 30mg / mL, hyaluronic acid acid 10μg / mL, grape seed extract 5mg / mL, ginsenoside 12mg / mL and tremella polysaccharide 10mg / mL.
[0054] Wherein, the first cytokine sustained-release sphere is prepared by the following method:
[0055] (1) adding dextran and polyethylene glycol into water respectively, and stirring until dissolved to obtain a 10wt% dextran aqueous solution and a 10wt% polyethylene glycol aqueous solution;
[0056] (2) At 0-4°C, mix dextran aqueous solution and polyethylene glycol aqueous solution at a mass ratio of 1:10, and then add interleukin-...
Embodiment 3
[0070] A hematopoietic stem cell culture medium, including basal medium and additives, the basal medium is DMEM medium, and the additives include the following components in terms of final concentration: trehalose 70ng / mL, vitamin C55ng / mL, berberine 2mg / mL, Stephanine 38μmol / mL, the first cytokine sustained-release sphere 2.0mg / mL, the second cytokine sustained-release sphere 22mg / mL, sodium chloride 4mg / mL, glutathione 25mg / mL, hyaluronic acid acid 6μg / mL, grape seed extract 3mg / mL, ginsenoside 7mg / mL and tremella polysaccharide 6mg / mL.
[0071] Wherein, the first cytokine sustained-release sphere is prepared by the following method:
[0072] (1) adding dextran and polyethylene glycol into water respectively, and stirring until dissolved to obtain a 10wt% dextran aqueous solution and a 10wt% polyethylene glycol aqueous solution;
[0073] (2) At 0-4°C, mix dextran aqueous solution and polyethylene glycol aqueous solution at a mass ratio of 1:8, and then add interleukin-3, i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com