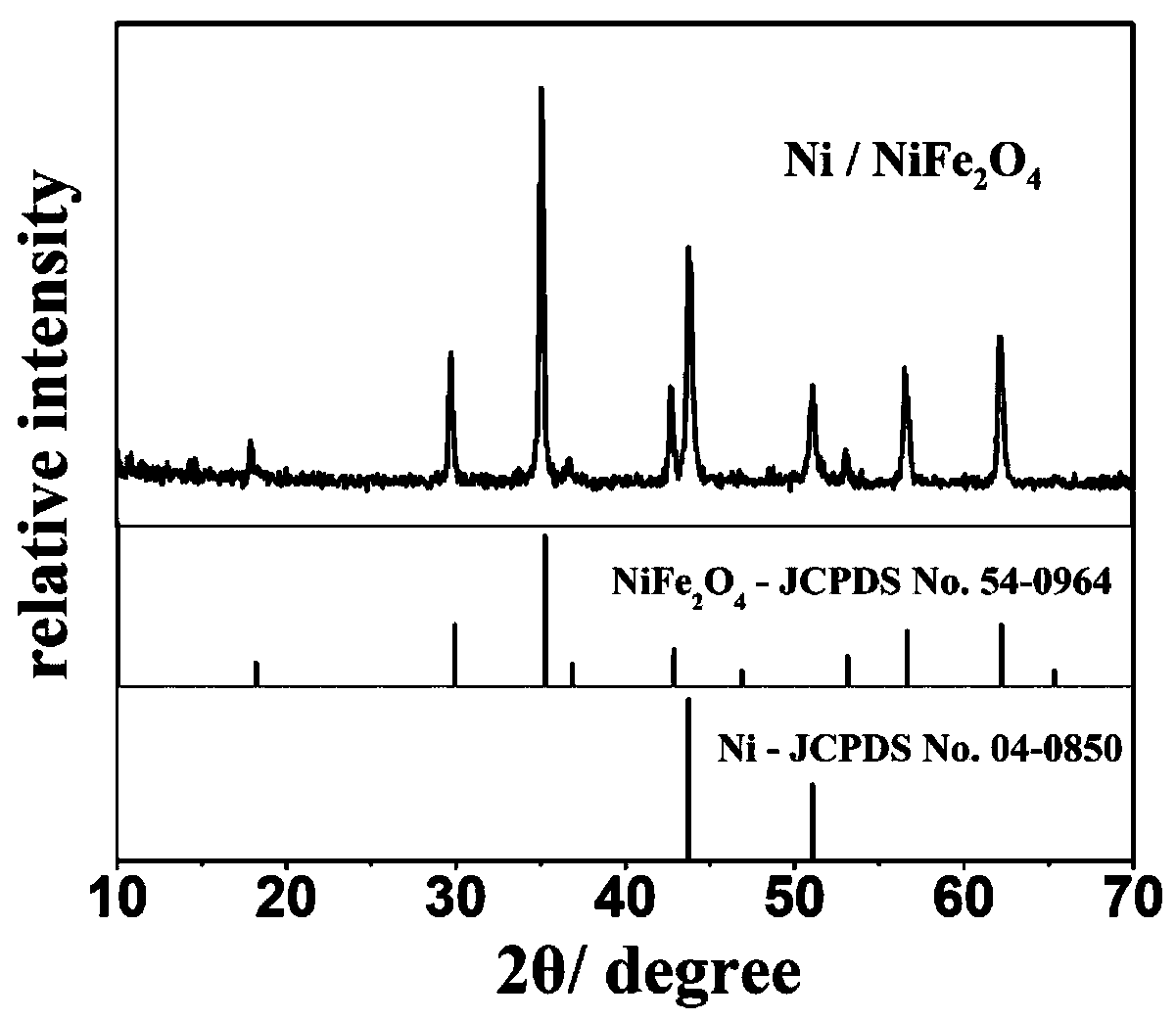
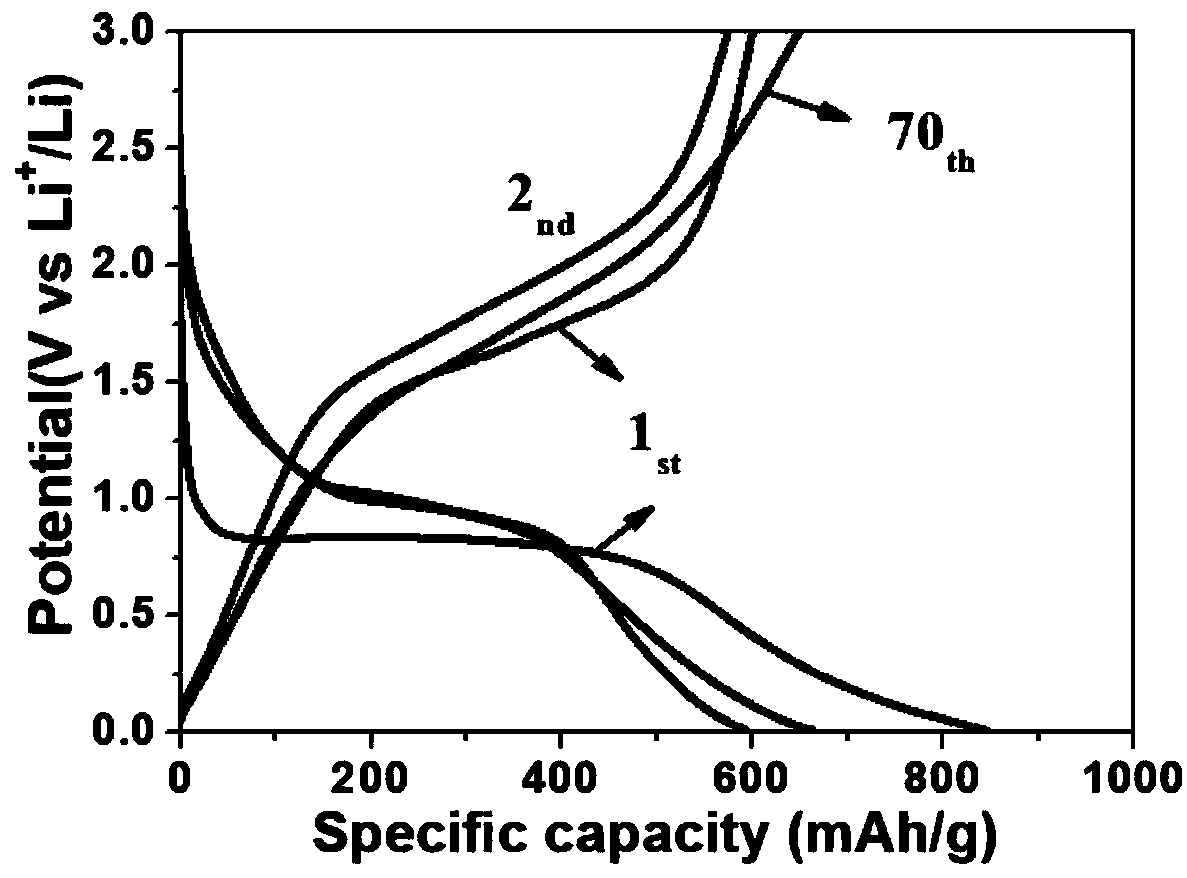
Ni/NiFe2O4 lithium ion battery anode material synthesized by bimetallic MOF precursor and preparation method thereof
A technology for lithium-ion batteries and negative electrode materials, applied in battery electrodes, secondary batteries, circuits, etc., can solve the problems of poor cycle performance and low conductivity, and achieve the effects of low cost, low equipment requirements, and simple synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] (1) Dissolve 0.727g of nickel nitrate hexahydrate and 1.352g of ferric chloride hexahydrate in 50mL of DMF respectively, and stir magnetically for 1h to form a uniform and stable solution; then add 0.453g of aminoterephthalic acid and 6mL of Sodium hydroxide solution, wherein the concentration of sodium hydroxide is 0.4moL / L.
[0027] (2) Then transfer the solution to a high-pressure reactor with a polytetrafluoroethylene liner, and place the reactor at 120° C. for 24 hours. After the reaction was completed, cool to room temperature, centrifuge, wash (three times with DMF), and dry at 80°C for 12 hours to obtain a brown Ni-Fe-MOFs precursor.
[0028] (3) Place the obtained brown Ni-Fe-MOFs precursor in a muffle furnace, raise the temperature to 500°C at 5°C / min, keep it warm for 2h, and then cool to room temperature to finally obtain Ni / NiFe 2 o 4 composite material.
Embodiment 2
[0030] (1) Dissolve 1.454g of nickel nitrate hexahydrate and 2.704g of ferric chloride hexahydrate in 50mL of DMF respectively, and magnetically stir for 1h to form a uniform and stable solution; then add 0.906g of aminoterephthalic acid and 12mL of Sodium hydroxide solution, wherein the concentration of sodium hydroxide is 0.4moL / L.
[0031](2) Then transfer the solution to a high-pressure reactor with a polytetrafluoroethylene liner, and place the reactor at 120° C. for 24 hours. After the reaction was completed, cool to room temperature, centrifuge, wash (three times with DMF), and dry at 80°C for 12 hours to obtain a brown Ni-Fe-MOFs precursor.
[0032] (3) Place the obtained brown Ni-Fe-MOFs precursor in a muffle furnace, raise the temperature to 500°C at 5°C / min, keep it warm for 2h, and then cool to room temperature to finally obtain Ni / NiFe 2 o 4 composite material.
Embodiment 3
[0034] (1) Dissolve 0.727g of nickel nitrate hexahydrate and 1.352g of ferric chloride hexahydrate in 50mL of DMF respectively, and stir magnetically for 1h to form a uniform and stable solution; then add 0.453g of aminoterephthalic acid and 6mL of Sodium hydroxide solution, wherein the concentration of sodium hydroxide is 0.4moL / L.
[0035] (2) Then transfer the solution to a high-pressure reactor with a polytetrafluoroethylene liner, and place the reactor at 120° C. for 24 hours. After the reaction was completed, cool to room temperature, centrifuge, wash (three times with DMF), and dry at 80°C for 12 hours to obtain a brown Ni-Fe-MOFs precursor.
[0036] (3) Place the obtained brown Ni-Fe-MOFs precursor in a muffle furnace, raise the temperature to 500°C at 5°C / min, keep it warm for 4h, and then cool to room temperature to finally obtain Ni / NiFe 2 o 4 composite material.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Size | aaaaa | aaaaa |
| Capacitance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com