Separation preparation and application of compound with antimicrobial activity to fungus secondary metabolic product
A compound and fermentation product technology, applied in the field of medicine, can solve problems such as increased medical expenses, increased mortality, and patient treatment failures
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
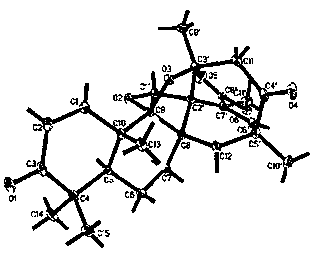
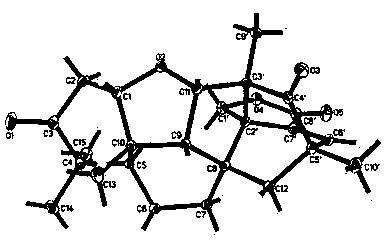
[0023] Example 1: Preparation and structure identification of compounds 1-5.
[0024] (1) Preparation of compound 1 as shown in formula (1)
[0025] 1. Fermentation conditions
[0026] Preparation of seed culture solution: Take 3.0g of yeast extract, 3.0g of malt extract, 5.0g of peptone, and 10.0g of glucose, dissolve them in an appropriate amount of water, then dilute to 1000mL with water, sterilize at 121°C for 30 minutes, and set aside. Aspergillus TJ23 is inoculated into the above-mentioned medium, and cultured on a shaker under the conditions of 25-28° C. and 100-120 rpm for 3-5 days to obtain a seed culture solution.
[0027] Fermentation: put 200g of rice into a 1000mL Erlenmeyer flask, add 200mL of water, sterilize at 121°C for 30min, and set aside. The above seed culture solution is inoculated into the rice culture medium, and cultured statically for 25-30 days under the condition of 25-28°C.
[0028] 2. Extraction and separation
[0029] The fermented product ob...
Embodiment 2
[0043] Example 2: See Table (3) for the antibacterial activity of compounds 1-5.
[0044] Table (3). Antibacterial activity of compounds 1-5 (MIC, μg / mL)
[0045]
[0046] a MRSA = methicillin-resistant Staphylococcus aureus ATCC 43300; b S. aureus = Staphylococcus aureus ATCC25923; c E. faecalis = Enterococcus faecalis ATCC 29212; d ESBL-E.coli=Escherichia coli ATCC 35218 producing extended-spectrum β-lactamase; e E.coli = Escherichia coli ATCC25922; f P. aeruginos = Pseudomonas aeruginosa ATCC 15442; g K. pneumonia = Klebsiella pneumoniae ATCC 700603; h The selection of recruiting product refers to CLSI standard (National Committee for Clinical Laboratory Standards, CLSI); Va=vancomycin; Ch=chloramphenicol; Am=amikacin; Ce=ceftriaxone.
[0047] Drug-resistant strains and control substances: The drug-resistant strains tested for antibacterial activity were purchased from the ATCC standard strain library, and the selection of control substances was in accordance with...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com