Method for preparing butene and butadiene by catalytic dehydrogenation of butane
A technology for catalytic dehydrogenation and butadiene, applied in chemical instruments and methods, catalyst activation/preparation, catalysts, etc., can solve problems such as deactivation, easy carbon deposition, environmental pollution, etc., and achieve great industrial value and application prospects, Good stability and reproducibility, environmentally friendly effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
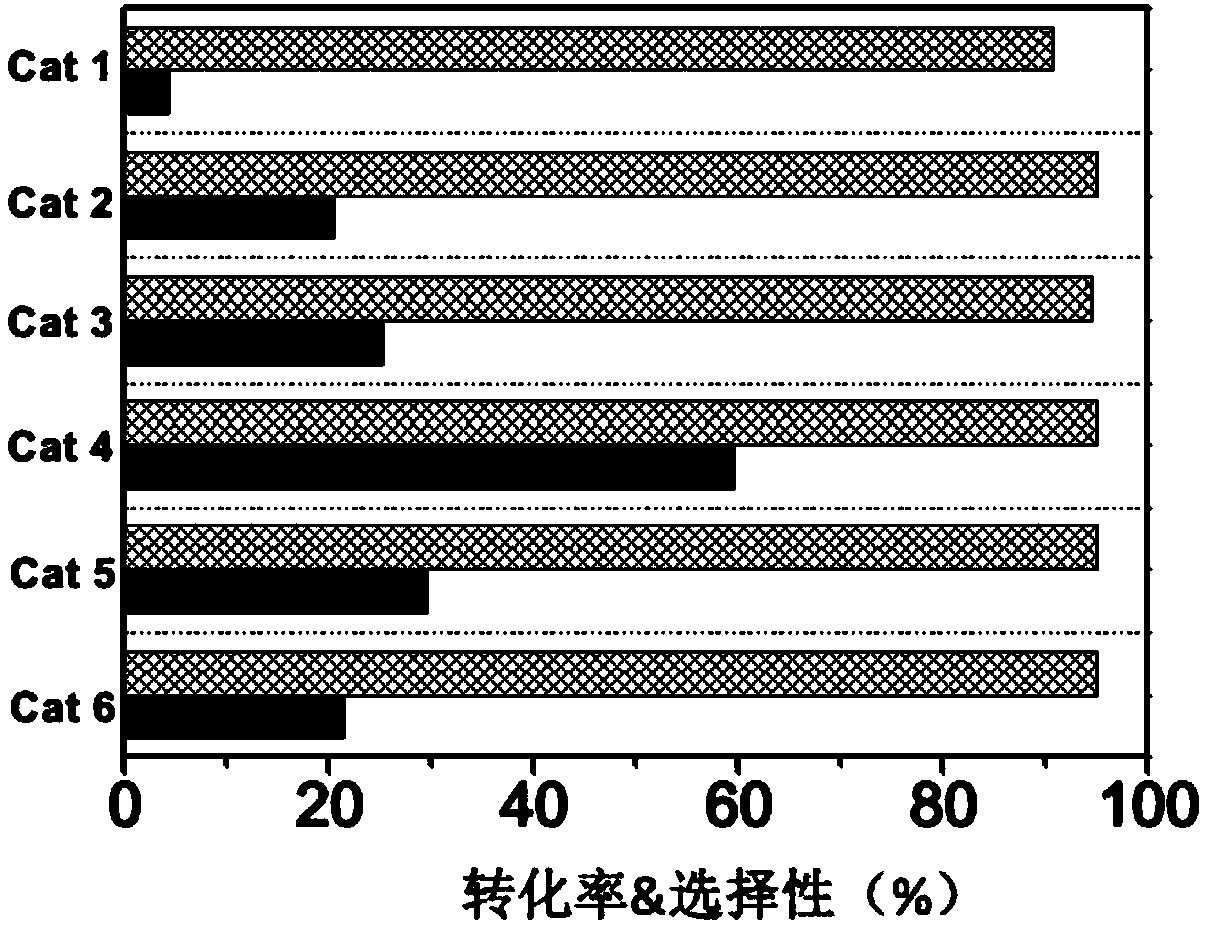
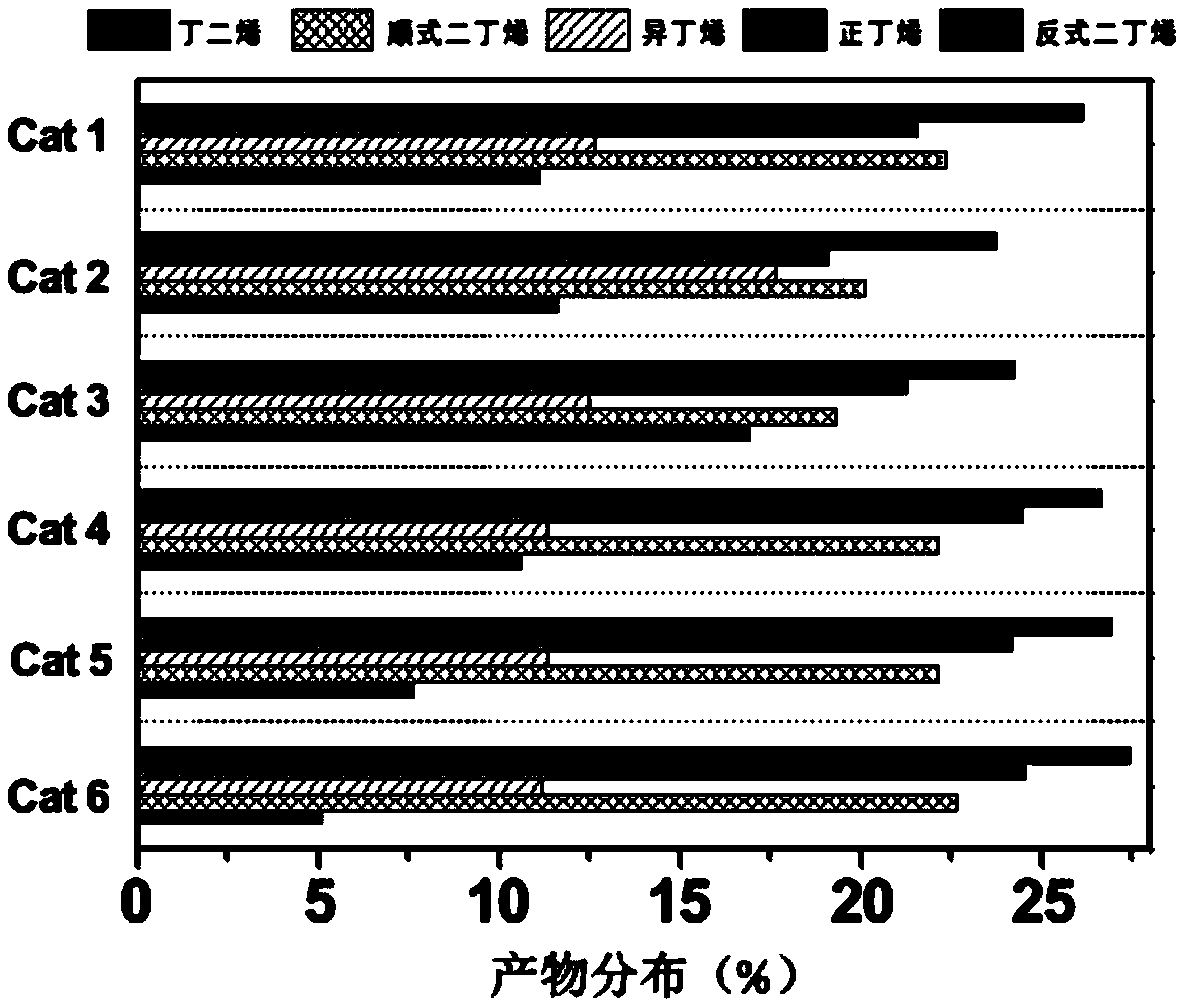
[0033] A certain amount of zirconium nitrate was weighed and prepared into a solution, and then impregnated with equal volume on the silicon-aluminum composite carrier. After impregnation, it was aged for 1 hour and then dried overnight in an oven at 100°C. The obtained catalyst was calcined at 750°C for 2 hours to obtain catalyst cat 1.
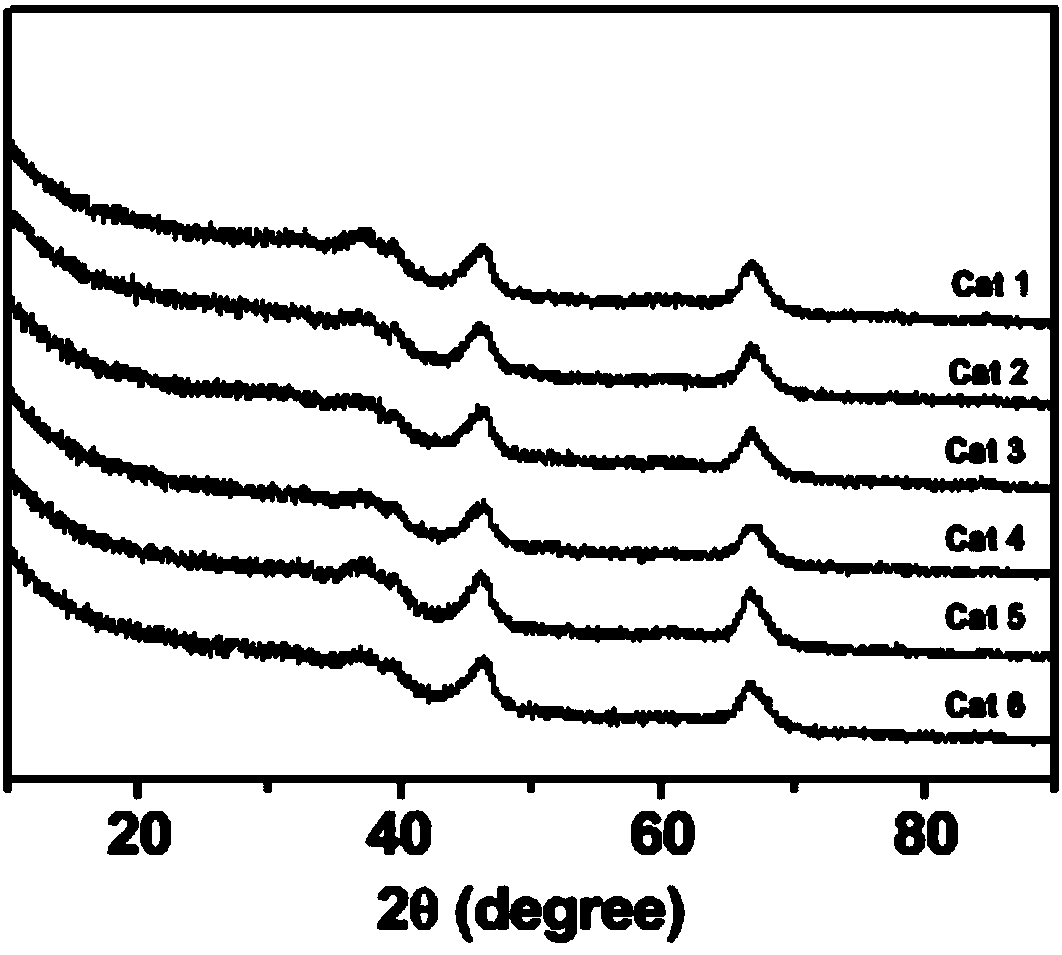
[0034] The mass fraction of zirconium in the catalyst obtained in the present embodiment is 4.47%. The specific surface area, pore volume and pore diameter of the catalyst are as shown in Table 1, and the X-ray diffraction pattern is as shown in Table 1. figure 1 shown.
Embodiment 2
[0036] According to the mass ratio of metal zirconium and metal gallium being 3.5:1, a certain amount of zirconium and gallium precursors, zirconium nitrate and gallium nitrate, were weighed and prepared into a mixed solution, and then impregnated in equal volume on the silicon-aluminum composite carrier. After impregnation, it was aged for 1 hour and then dried overnight in an oven at 100°C. The obtained catalyst was calcined at 750°C for 2 hours to obtain catalyst cat 2.
[0037]The mass fraction of zirconium in the catalyst obtained in this example was 3.53%, and the mass fraction of gallium was 0.81%. The specific surface area, pore volume and pore diameter of this catalyst are as shown in Table 1, and the X-ray diffraction figure is as follows figure 1 shown.
Embodiment 3
[0039] According to the mass ratio of metal zirconium and metal gallium as 2:2.5, a certain amount of zirconium and gallium precursors zirconium nitrate and gallium nitrate were weighed and prepared into a mixed solution, and then impregnated in equal volume on the silicon-aluminum composite carrier. After impregnation, it was aged for 1 hour and then dried overnight in an oven at 100°C. The obtained catalyst was calcined at 750°C for 2 hours to obtain catalyst cat 3.
[0040] The mass fraction of zirconium in the catalyst obtained in this example was 1.89%, and the mass fraction of gallium was 2.23%. The specific surface area, pore volume and pore diameter of this catalyst are as shown in Table 1, and the X-ray diffraction figure is as follows figure 1 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com