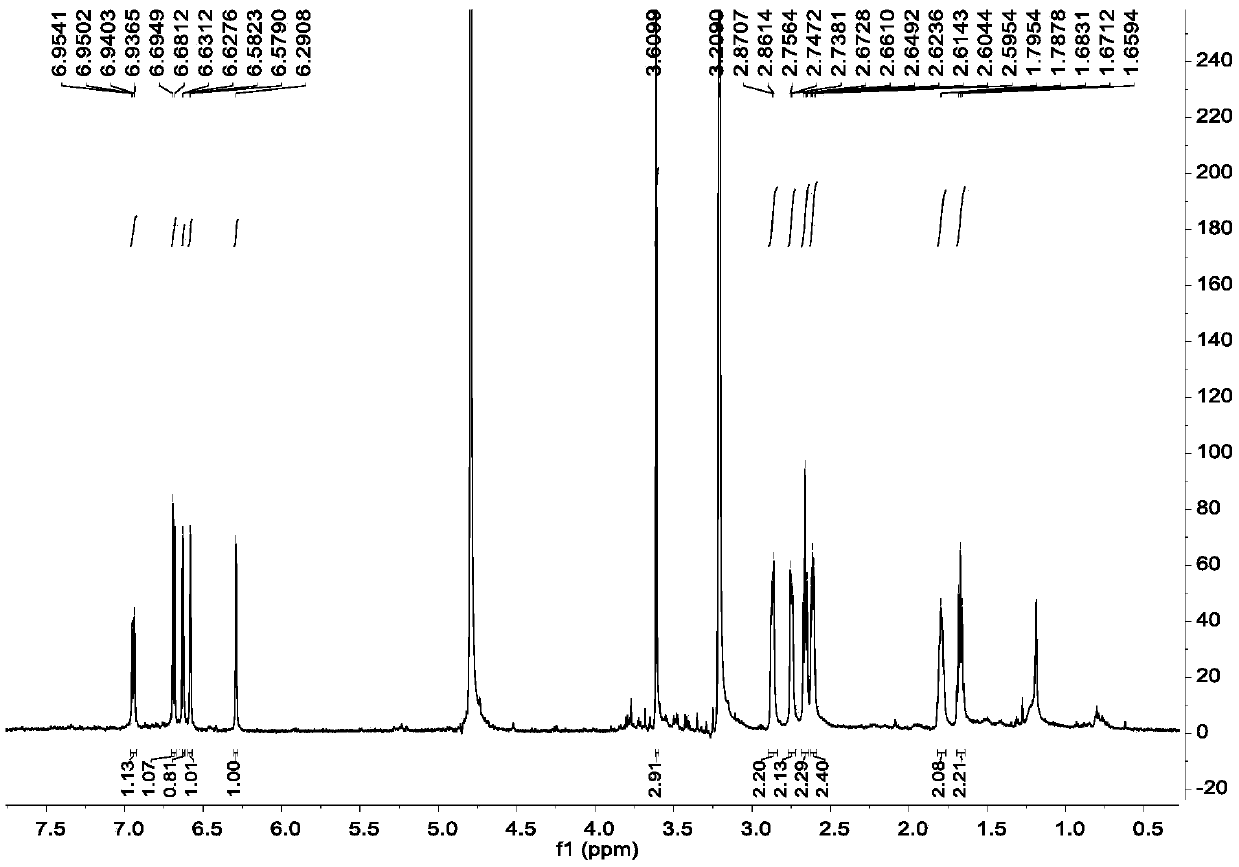
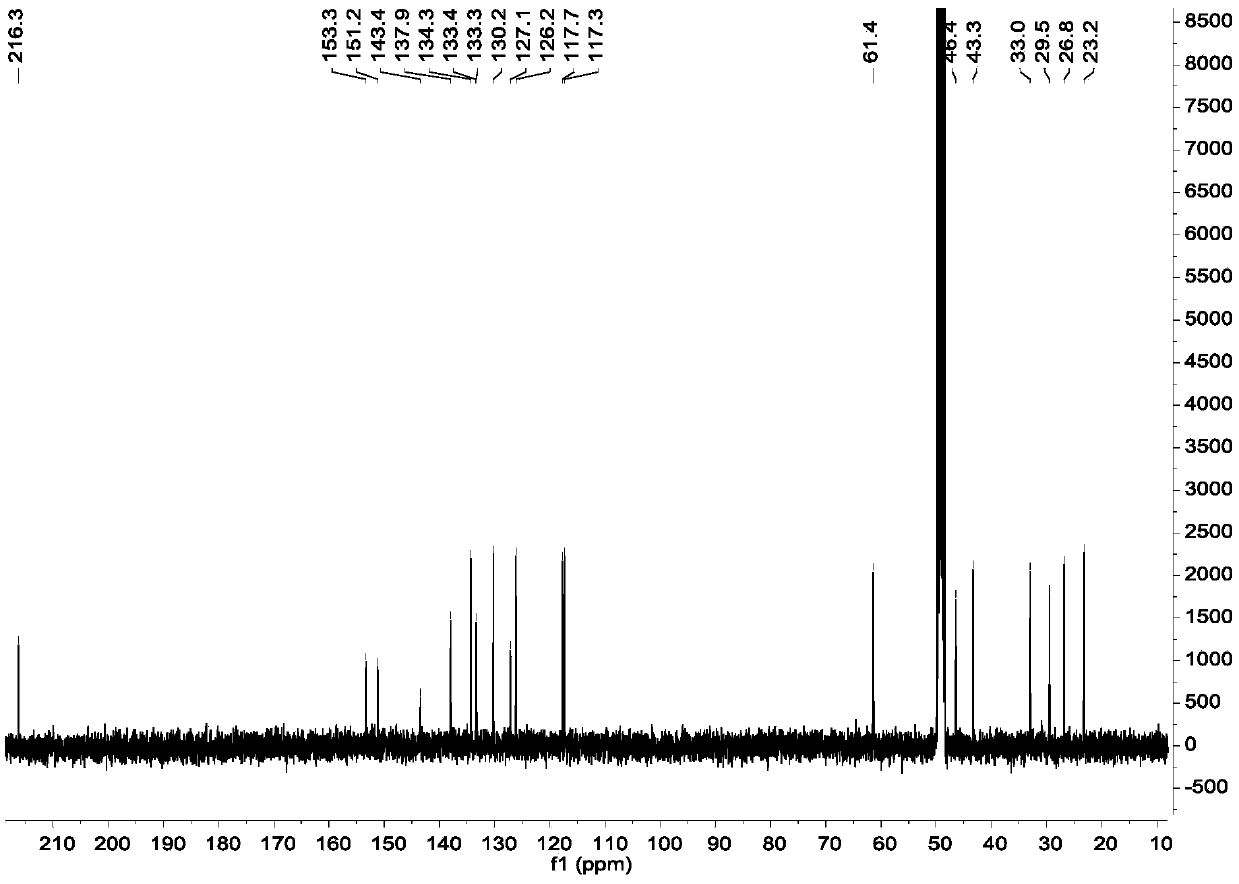
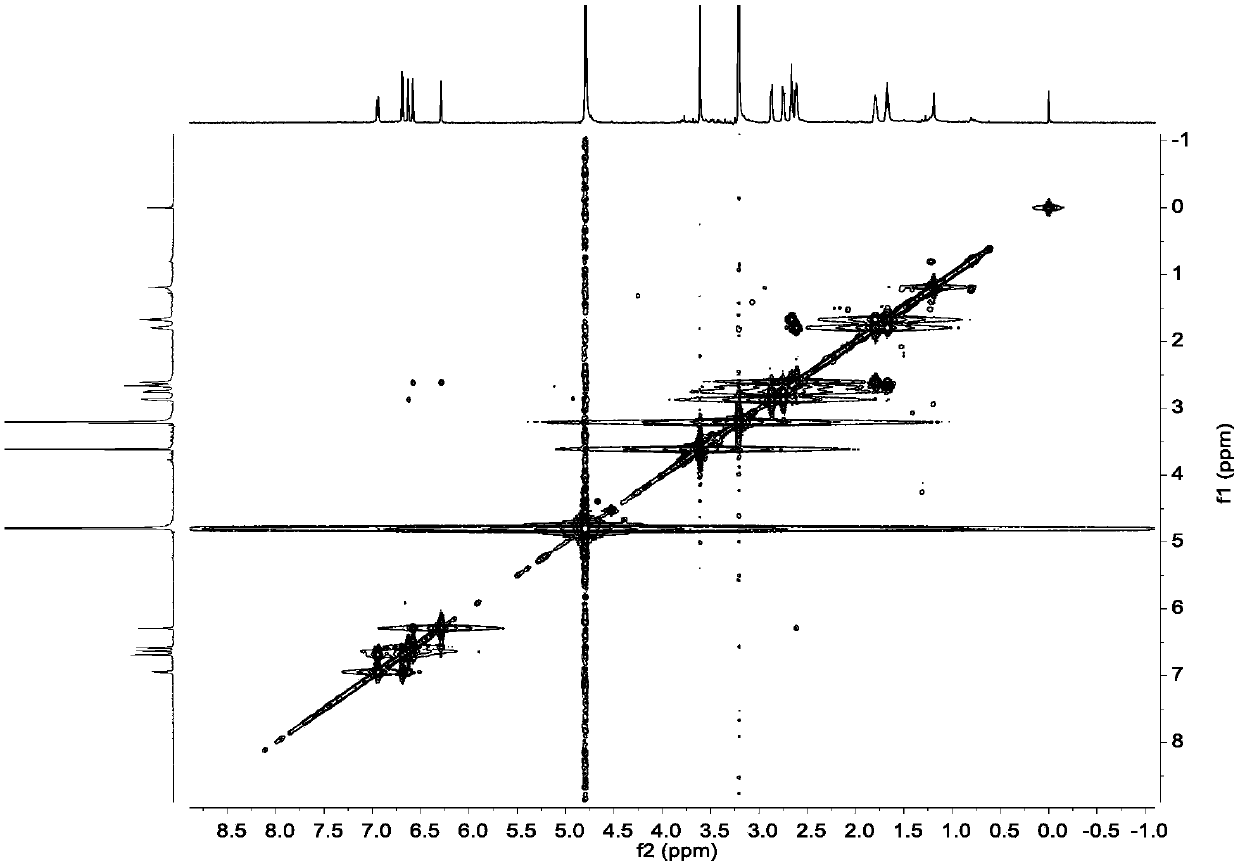
Diarylheptanoids extracted from epicarp of immature fruits of juglans mandshurica and juglans regia as well as preparation method and application of diarylheptanoids
A technology of diaryl heptane and compounds, applied in the field of organic compounds, can solve the problems of high price, large side effects, and easy drug resistance caused by long-term application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Embodiment 1, the preparation method of Qinglongyi ethanol extract extract
[0052] Dry Qinglongyi, take 10kg of dried Qinglongyi medicinal material, pulverize, soak in 95vol% ethanol aqueous solution, heat and reflux extract 4 times, each time for 3 hours, to obtain ethanol extract, and concentrate under reduced pressure to obtain 611g of ethanol extract extract.
Embodiment 2
[0053] Example 2, Separation and Purification of Diaryl Heptane Monomer Compounds in Qinglongyi Ethanol Extract Extract
[0054] Instrument materials: silica gel (200-300 mesh) for column chromatography, silica gel GF for thin layer chromatography 254 (10-40μm) (manufactured by Qingdao Ocean Chemical Co., Ltd.), column chromatography ODS: YMC*GEL ODS-A-HG (50μm), Sephadex LH-20 for column chromatography (Beijing Jinouya Technology Development Co., Ltd.), column chromatography With HW-40C: Japan, semi-preparative chromatographic column: COSMOSIL 5C18-MS-II 10ID×250mm.
[0055] Separation and purification process: the ethanol extract prepared in Example 1 is subjected to silica gel column chromatography, and the volume ratio is 100:0, 100:0.5, 100:1, 100:2, 100:3, 100:4, 100 :5, 100:6, 100:8, 100:10, 100:12, 100:14, 100:16, 100:18, 100:20, 100:25, 100:30, 100:50, 100:100 , 0:100 petroleum ether-acetone system for gradient elution, after TLC detection, the samples with the sa...
Embodiment 4
[0082] Embodiment 4, the inhibitory effect of formula I~IV compound on PLC5 liver cancer cell
[0083] PLC5 liver cancer cells are formed from malignant liver epithelial cells and are a common type of liver cancer in clinic.
[0084] The revived PLC5 cells were expanded and cultured until the number reached the experimental requirement. Inoculate 2000 PLC5 liver cancer cells per well on a 96-well plate. After 24 hours, the cells grew attached to the wall, and different concentrations of drugs were added to each well, and the cell morphology was continuously observed. After 48 hours, the absorbance value (OD value) of each well was measured at a wavelength of 450 nm with a microplate reader.
[0085] The results show that the half-inhibitory concentrations of the compounds represented by formulas I, II, III, and IV to PLC5 liver cancer cells are 80.5 μM, 448.7 μM, 92.6 μM, and 121.2 μM, respectively, indicating that these compounds have certain inhibition on the growth of PLC5...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Half inhibitory concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com