Organic small molecule containing boron-nitrogen coordination bond, and preparation method and sensing application thereof to fluorine ion
A technology of coordination bonds and small molecules, applied in the field of small organic molecules and their preparation, can solve the problems of low fluorescence luminescence efficiency of fluoride ion probe materials, low fluoride ion response value, poor fluorine ion selectivity, etc., to achieve Photochemical stability, easy operation, good selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0039] 2. Preparation of fluorescent probes
[0040] 1) Dissolve the fluorescent compound in tetrahydrofuran to prepare 1×10 -3 stock solution of M;
[0041] 2) Add 4950 μL tetrahydrofuran to every 50 μL stock solution, and leave it at room temperature for 2 hours to prepare the fluorescent probe.
[0042] For detection: use 1.0M tetrabutylammonium fluoride tetrahydrofuran solution to prepare 1×10 -3 M is the solution of fluoride ion to be measured.
Embodiment 1
[0044] Under argon protection, compound 1 (0.478g, 1.38mmol) was dissolved in absolute anhydrous THF (10mL), and n-butyllithium (0.56mL, 2.5M in hexane, 1.38mmol) was added dropwise at -78°C , kept for 1h after the dropwise addition, dissolved triphenylboron (0.388g, 1.6mmol) in absolute anhydrous THF (10mL), gradually added dropwise to the above solution under the protection of argon, then warmed up to room temperature, continued The reaction was stirred for 12 h, the solvent was removed under reduced pressure, and a yellow solid was obtained by separation and purification on a silica gel column, the yield: 35%.
[0045] The H NMR spectrum of the obtained product is as Figure 6 As shown, the carbon NMR spectrum is as Figure 7 shown.
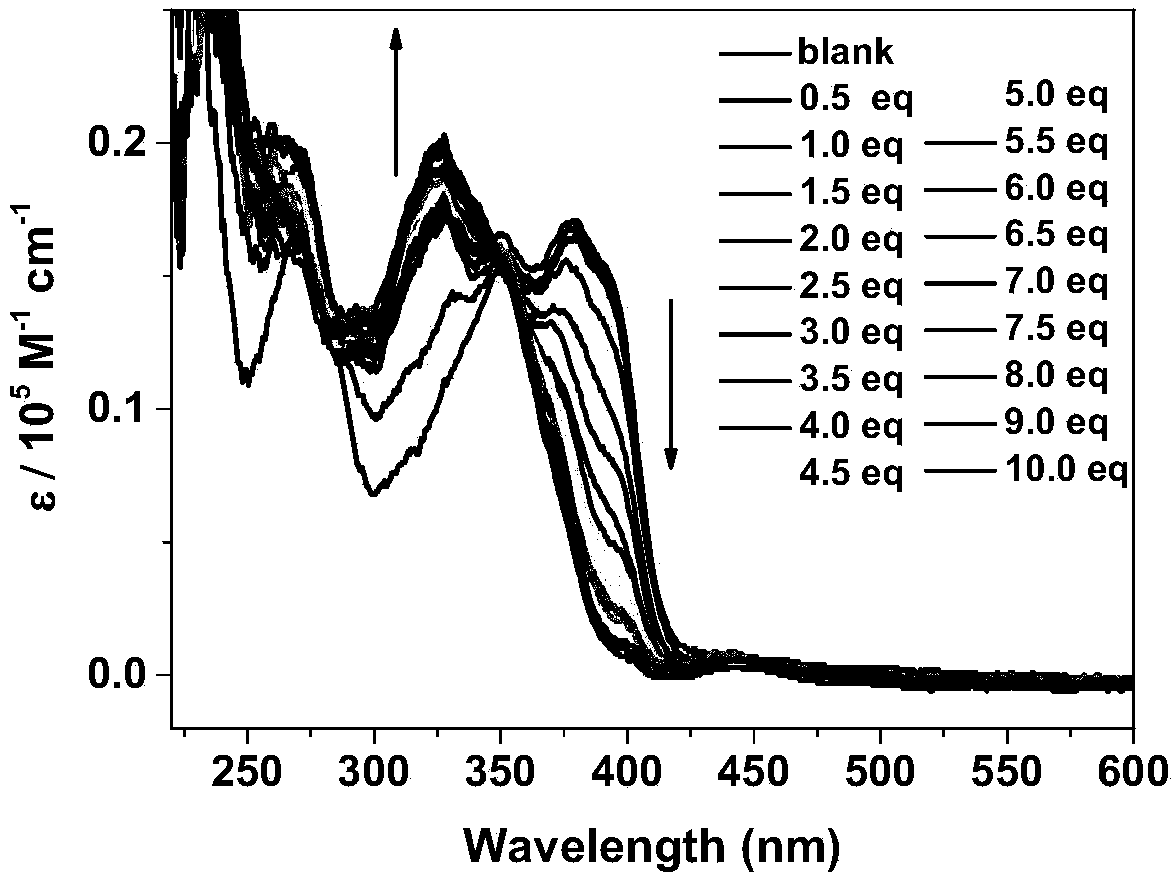
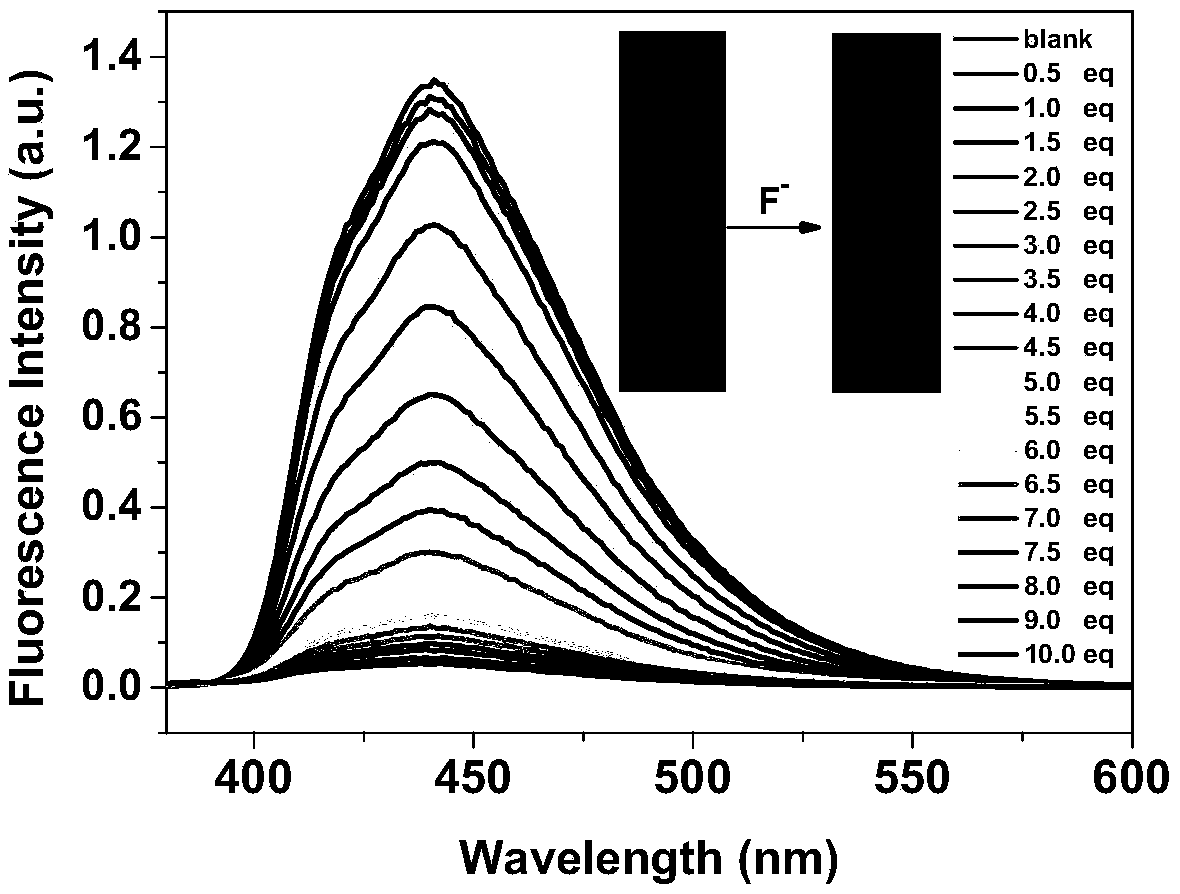
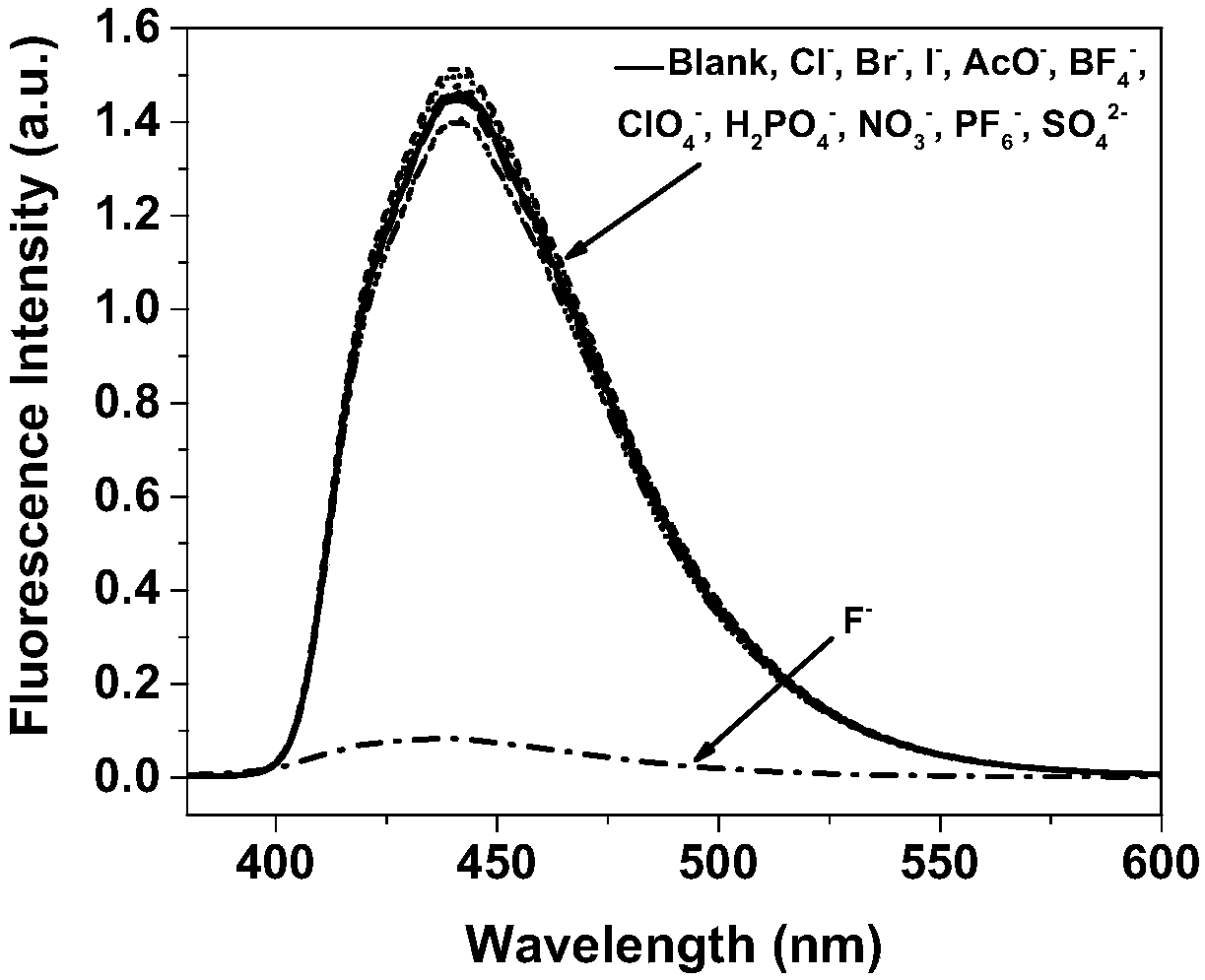
[0046] In order to verify the effect of the present invention, the detection of fluoride ions by the boron nitrogen compound synthesized in Example 1 was explored, as shown in the figure.
[0047] in, figure 1 , figure 2 is the UV-visible ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com