Detection method of pimavanserin tartrate
A technology of pimavanserin and detection method, which is applied in the field of drug analysis and achieves the effects of reliable method, high precision and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Pimavanserin tartrate purity test:
[0039] 1) Prepare the test sample solution:
[0040] Accurate 25mg of pimavanserin tartrate standard substance, in a 20mL volumetric flask, add an appropriate amount of diluent to dissolve and dilute to the mark, mix well, and use it as the test sample solution;
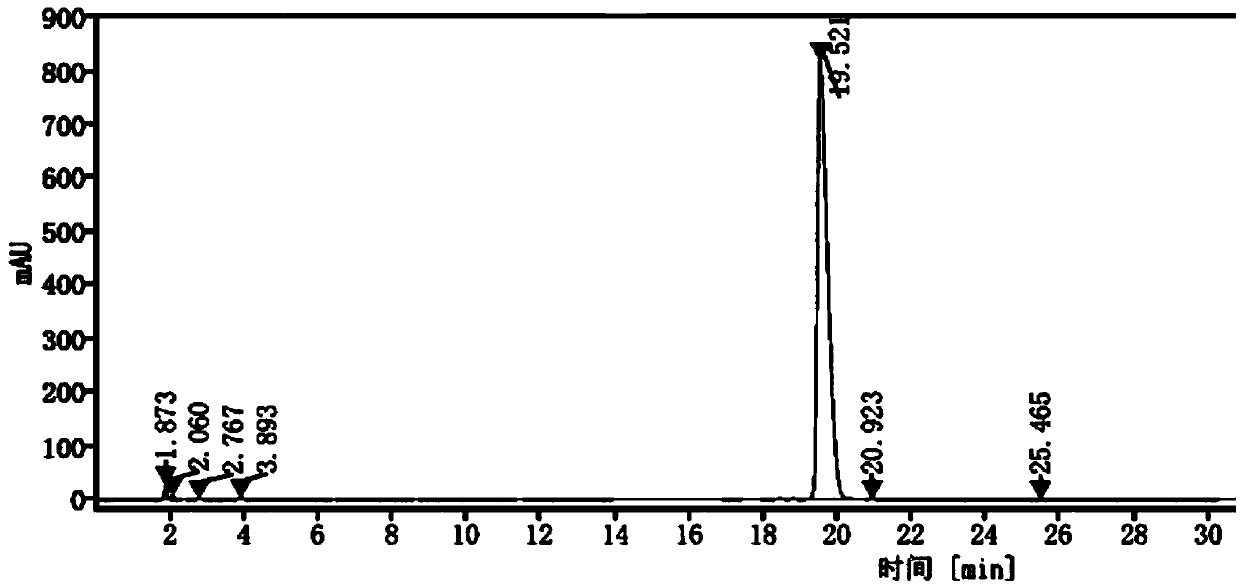
[0041] 2) High performance liquid chromatography determination: determination conditions are: Gensial CN chromatographic column: 4.6mm×250mm, 5μm; mobile phase is acetonitrile: potassium dihydrogen phosphate solution = (45~70): (55~30) (v / v ) for gradient elution, the order of gradient elution time and the volume ratio of mobile phase acetonitrile is: 0-7min 45% operation, 7-12min from 45%-30% operation, 12-20min from 30%-70% operation, 20 ~30min 70% operation; flow rate is 1.0mL / min; column temperature is 25°C; UV detector detection wavelength is 215nm; injection volume is 10μL, record the chromatogram, see figure 1 ;
[0042] Depend on figure 1 It can be seen that the...
Embodiment 2
[0044] Pimavanserin tartrate system adaptability study:
[0045] 1) Prepare the test sample solution:
[0046] Accurately take by weighing pimavanserin tartrate standard substance 24.98mg in the 20mL volumetric flask, add diluent to dissolve and dilute to the mark, mix, and the concentration of pimavanserin tartrate is 1.2440mg / mL for the test sample solution ;
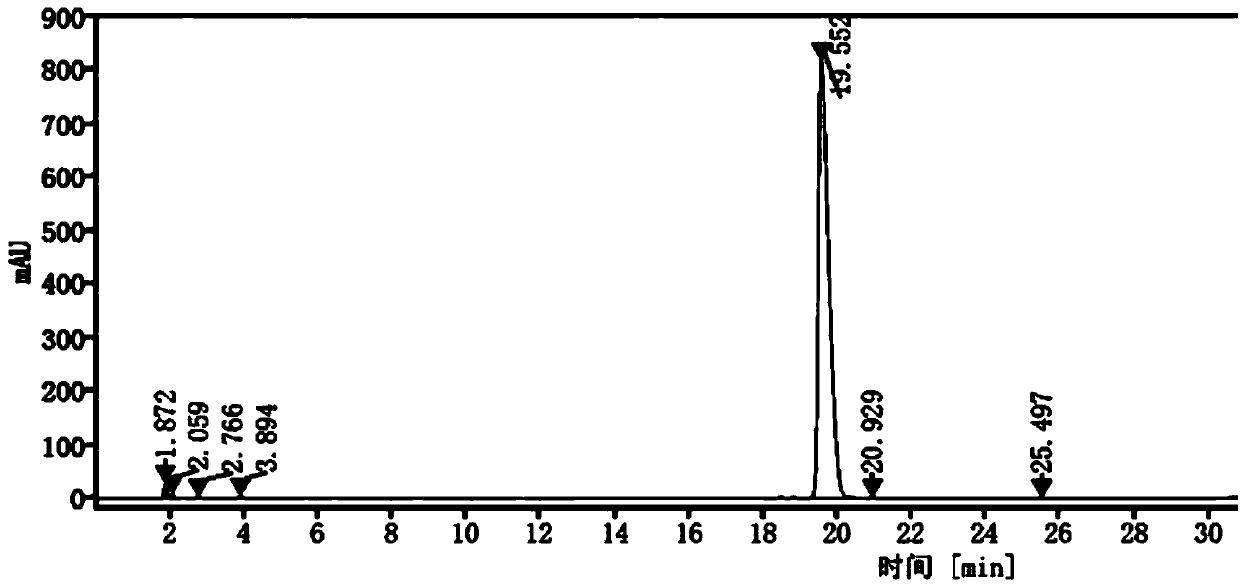
[0047] 2) High-performance liquid chromatography measurement: The measurement conditions are: Gensial CN chromatographic column: 4.6mm×250mm, 5μm; mobile phase is acetonitrile: potassium dihydrogen phosphate solution = (45:70) ~ (55:30) (v / v ) for gradient elution, the order of gradient elution time and the volume ratio of mobile phase acetonitrile is: 0-7min 45% operation, 7-12min from 45%-30% operation, 12-20min from 30%-70% operation, 20 ~30min 70% operation; flow rate is 1.0mL / min; column temperature is 25°C; UV detector detection wavelength is 215nm; injection volume is 10μL, record the chromatogram (see figu...
Embodiment 3
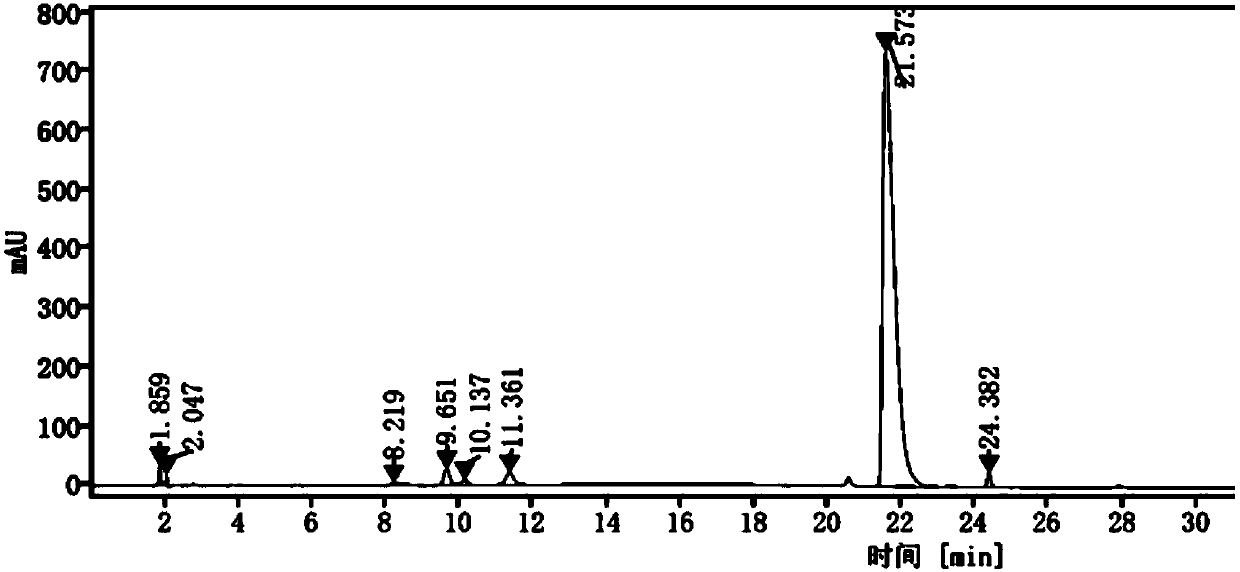
[0052] Investigation on the specificity of pimavanserin tartrate:
[0053] 1) Preparation of impurity stock solution: Weigh 5.12 mg of impurity A, 5.17 mg of impurity B, 5.23 mg of impurity C, 5.16 mg of impurity D, and 5.19 mg of impurity E in a 20mL volumetric flask, add diluent to dissolve and dilute to the mark, mix Uniform, as impurity stock solution stand-by;
[0054]Wherein impurity A is 4-isobutoxybenzonitrile, impurity B is 4-isobutoxybenzylamine, impurity C is 4-(4-fluorobenzylamino)-1-methylpiperidine, and impurity D is 1,3-bis(4-isobutoxybenzyl)urea, impurity E is 1-(4-fluorobenzyl)-3-(4-isobutoxybenzyl)urea;
[0055] 2) Resolution solution preparation: Weigh 25.04 mg of pimavanserin tartrate standard substance into a 20 mL volumetric flask, add 1 mL each of the above-mentioned impurity stock solution, dissolve with diluent and dilute to the mark, mix well, and use it as the resolution Solution (wherein, the pimavanserin tartrate standard solution concentration i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com