A kind of detection method of pimavanserin tartrate
A technology of pimavanserin and detection method, which is applied in the field of drug analysis, and achieves the effect of reliable method, good stability and good specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038]Purity test of pimofanserin tartrate:
[0039]1) Prepare test sample solution:
[0040]Accurate 25mg pimofenserin tartrate standard, in a 20mL volumetric flask, add an appropriate amount of diluent to dissolve and dilute to the mark, mix well, as the test sample solution;
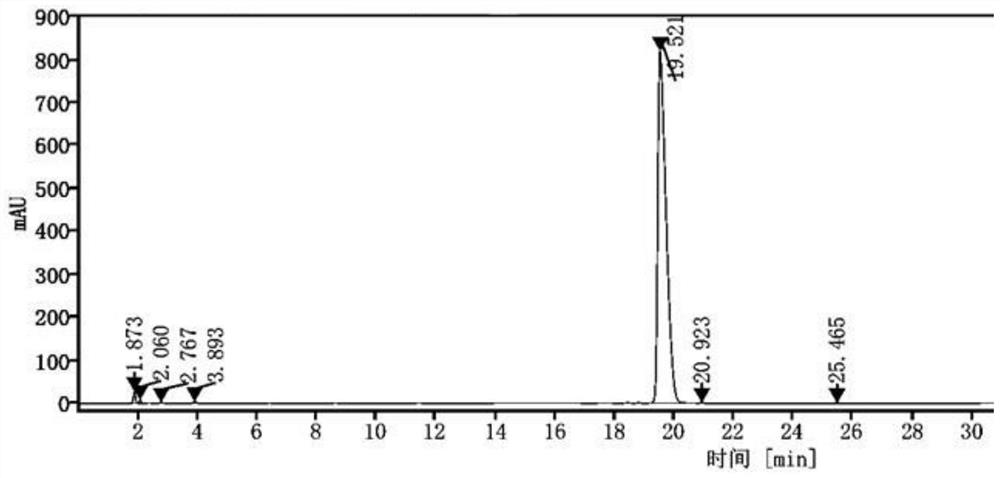
[0041]2) High performance liquid chromatography determination: determination conditions: Gensial CN column: 4.6mm×250mm, 5μm; mobile phase is acetonitrile: potassium dihydrogen phosphate solution = (45~70): (55~30)(v / v ) For gradient elution, the order of the gradient elution time and the volume ratio of mobile phase acetonitrile is: 0~7min 45% run, 7~12min run from 45%~30%, 12~20min run from 30%~70%, 20 ~30min 70% operation; flow rate is 1.0mL / min; column temperature is 25℃; UV detector detection wavelength is 215nm; injection volume is 10μL, record chromatogram, seefigure 1 ;
[0042]byfigure 1 It can be seen that the high performance liquid chromatogram of pimofanserin tartrate has good peak shape and high peak puri...
Embodiment 2
[0044]System adaptability study of pimofenserin pimobendan tartrate:
[0045]1) Prepare test sample solution:
[0046]Accurately weigh 24.98 mg of pimovanserin tartrate standard substance in a 20mL volumetric flask, add diluent to dissolve and dilute to the mark, and mix well to obtain a test sample solution with a concentration of pimovanserin tartrate of 1.2440 mg / mL ;
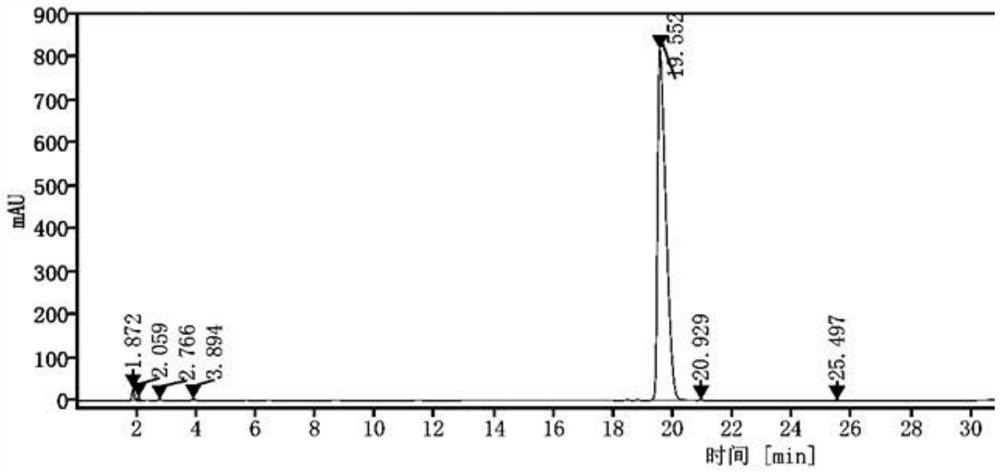
[0047]2) High performance liquid chromatography determination: the determination conditions are: Gensial CN column: 4.6mm×250mm, 5μm; mobile phase is acetonitrile: potassium dihydrogen phosphate solution = (45:70) ~ (55:30) (v / v ) For gradient elution, the order of gradient elution time and the volume ratio of mobile phase acetonitrile is: 0~7min 45% operation, 7~12min 45%~30%, 12~20min 30%~70%, 20 ~30min 70% operation; flow rate is 1.0mL / min; column temperature is 25℃; UV detector detection wavelength is 215nm; injection volume is 10μL, record chromatogram (seefigure 2 ), continuous measurement 6 times, the results are sh...
Embodiment 3
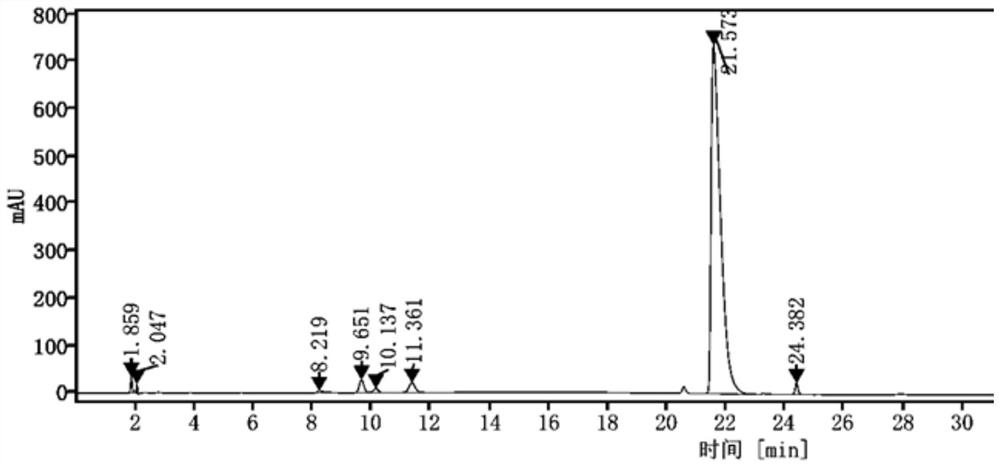
[0052]Investigation on the specificity of pimophanserin tartrate:
[0053]1) Preparation of impurity stock solution: Weigh impurity A 5.12mg, impurity B 5.17mg, impurity C 5.23mg, impurity D 5.16mg, impurity E 5.19mg into a 20mL volumetric flask, add diluent to dissolve and dilute to the mark, mix Evenly, as an impurity stock solution for later use;
[0054]Among them, impurity A is 4-isobutoxybenzonitrile, impurity B is 4-isobutoxybenzylamine, impurity C is 4-(4-fluorobenzylamino)-1-methylpiperidine, and impurity D is 1,3-bis(4-isobutoxybenzyl)urea, impurity E is 1-(4-fluorobenzyl)-3-(4-isobutoxybenzyl)urea;
[0055]2) Preparation of resolution solution: Weigh 25.04 mg of pimovanserin tartrate standard into a 20 mL volumetric flask, and add 1 mL of each of the above impurity stock solutions, dissolve and dilute to the mark with a diluent, and mix, as the resolution Solution (wherein, the concentration of pimofenserin tartrate standard solution in the solution is: 1.2520mg / mL, the concentrat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com