Kit and method for determination of glycosylated hemoglobin in whole blood
A technology of glycosylated hemoglobin and kits, which is applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems of complicating the measurement process and reducing the detection efficiency, and achieve the effect of avoiding changes, reducing complex processes, and accurate detection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
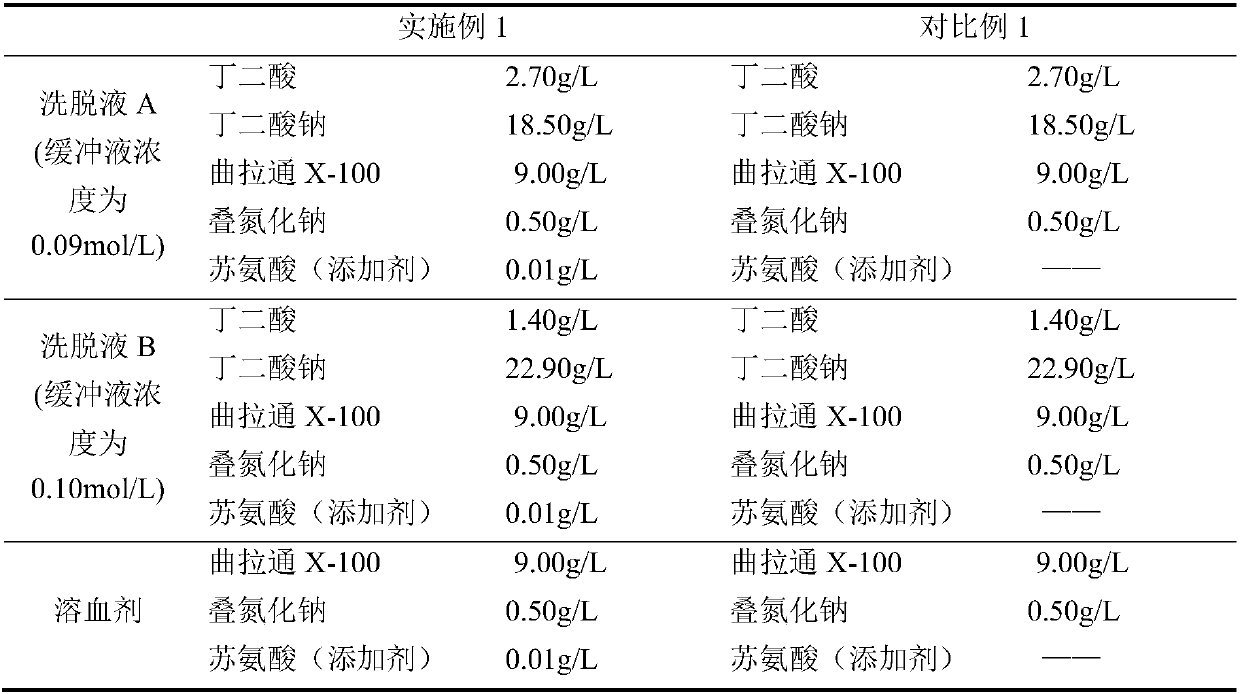
[0025] A kit for measuring glycosylated hemoglobin in whole blood, comprising eluent A, eluent B and hemolytic agent, eluent A and eluent B respectively include buffer, surfactant, preservative and additives, hemolytic The agent includes a surfactant, a preservative and an additive, and the additive is threonine.
[0026] The amount of additive added in eluent A, eluent B and hemolytic agent was all 0.01g / L.
[0027] The buffer is a succinate buffer, and the succinate buffer in the eluent A includes succinic acid with a mass volume concentration of 2.70 g / L and sodium succinate with a mass volume concentration of 18.50 g / L; The succinate buffer solution of eluent B includes succinic acid with a mass volume concentration of 1.40 g / L and sodium succinate with a mass volume concentration of 22.90 g / L.
[0028] The molar concentration of succinate buffer in eluent A is 0.09mol / L.
[0029] The molar concentration of succinate buffer in eluent B is 0.10mol / L.
[0030] The surfact...
Embodiment 2
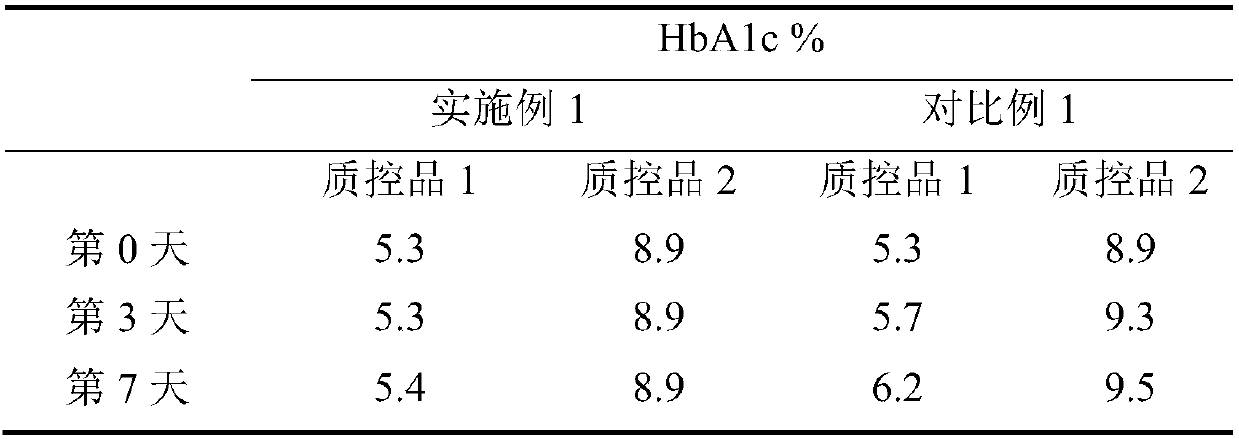
[0039] A method for measuring glycosylated hemoglobin in whole blood, comprising the following steps: add a hemolytic agent to the sample and then hemolyze, and the sample after hemolysis is eluted with an eluent in an analysis column to obtain HbA1c components and HbA0 components, and then perform Detect and calculate the detection result of HbA1c.
[0040] The sample is a quality control product, and the quality control product is lyophilized powder of glycated hemoglobin blood sample.
[0041] The HbA1c in the quality control product was detected by using the kits of Example 1 and Comparative Example 1, and the detection instrument was a glycated hemoglobin meter.
[0042] Take different quality control products, coded as quality control product 1 and quality control product 2. After the quality control product is reconstituted, the quality control product solution is obtained, and the volume ratio is 1:200. The hemolytic agent in Example 1 and Comparative Example 1 was ad...
Embodiment 3
[0049] The kit in Example 1 was used to detect HbA1c in the sample under the assay method provided in Example 2, wherein the samples were 24 cases of frozen whole blood samples used for traceability by IFCC, sampled from the Netherlands, and stored in dry ice throughout the air transport. The ambient temperature is not higher than -40°C, and then stored in a -80°C ultra-low temperature refrigerator. Before the sample test, return the frozen sample to room temperature, take 20 μL for each case, and add it according to the volume ratio of 1:200. The hemolytic agent of Example 1 performed hemolysis. It took 26 days from the sampling of the samples in the Netherlands to the detection test.
[0050] The test results are compared with the IFCC target values given by the IFCC standard laboratory, as shown in Table 3.
[0051] The detection result of the kit of table 3 embodiment 3 is compared with IFCC target value
[0052]
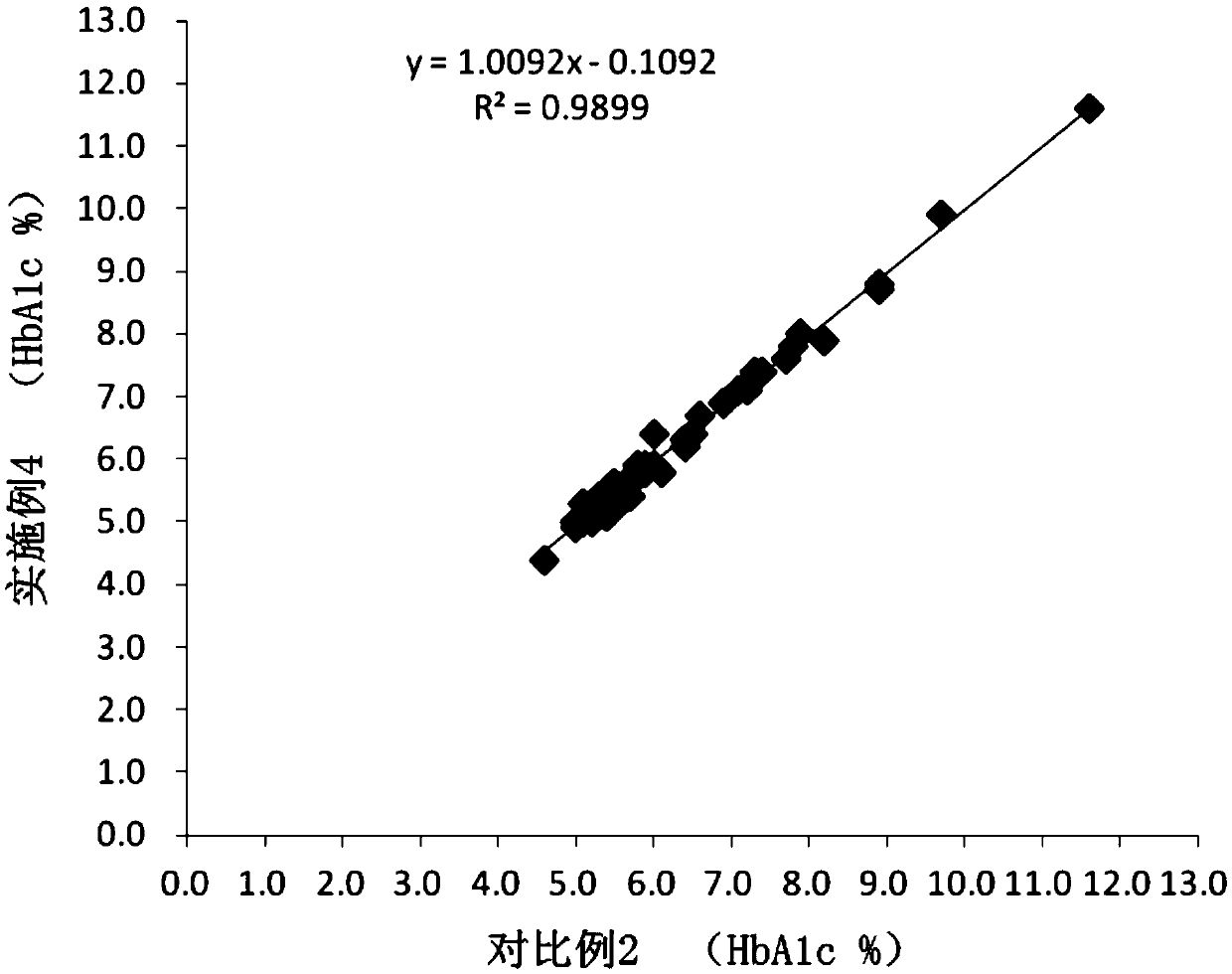
[0053] It can be seen from Table 3 that the HbA1c v...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com