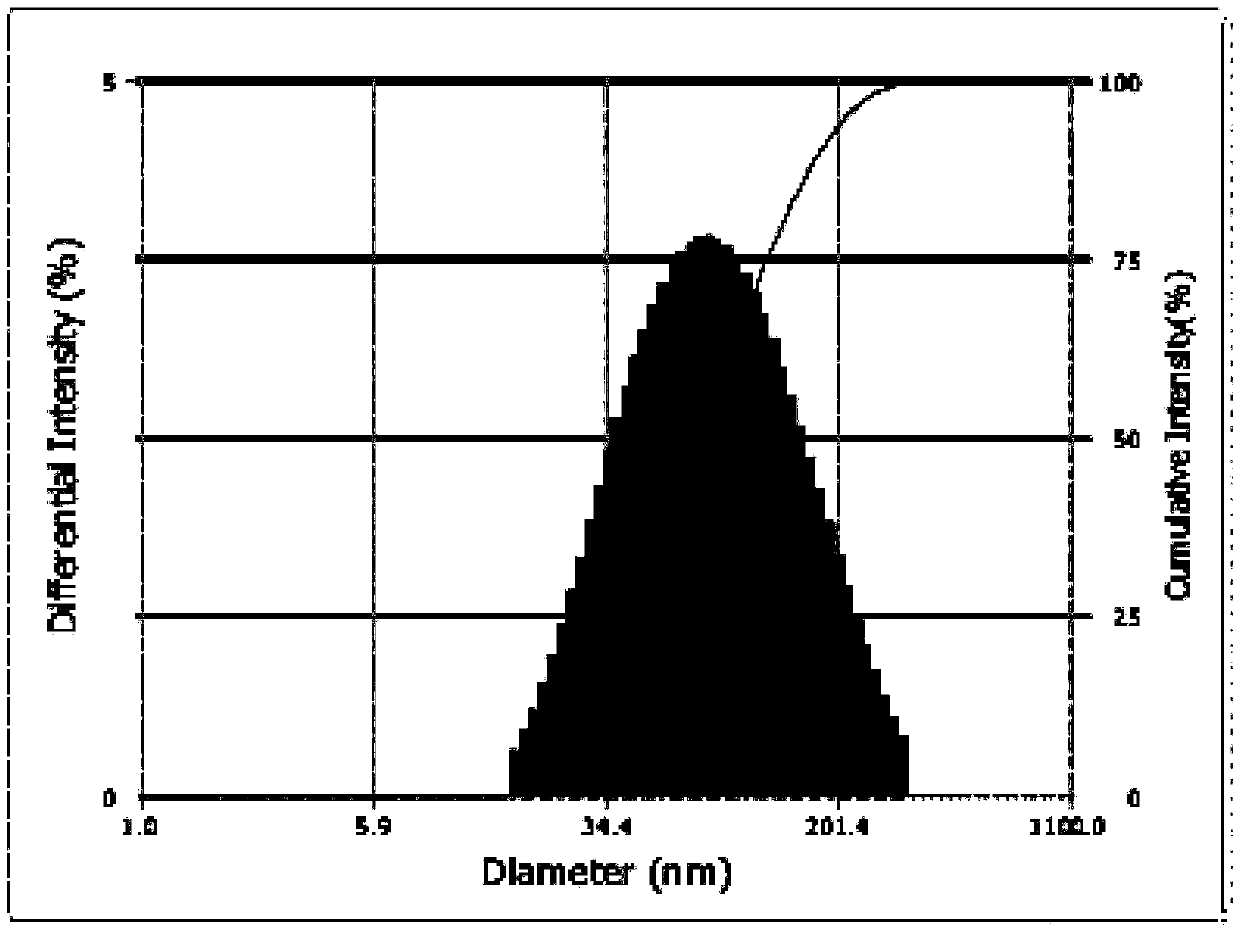
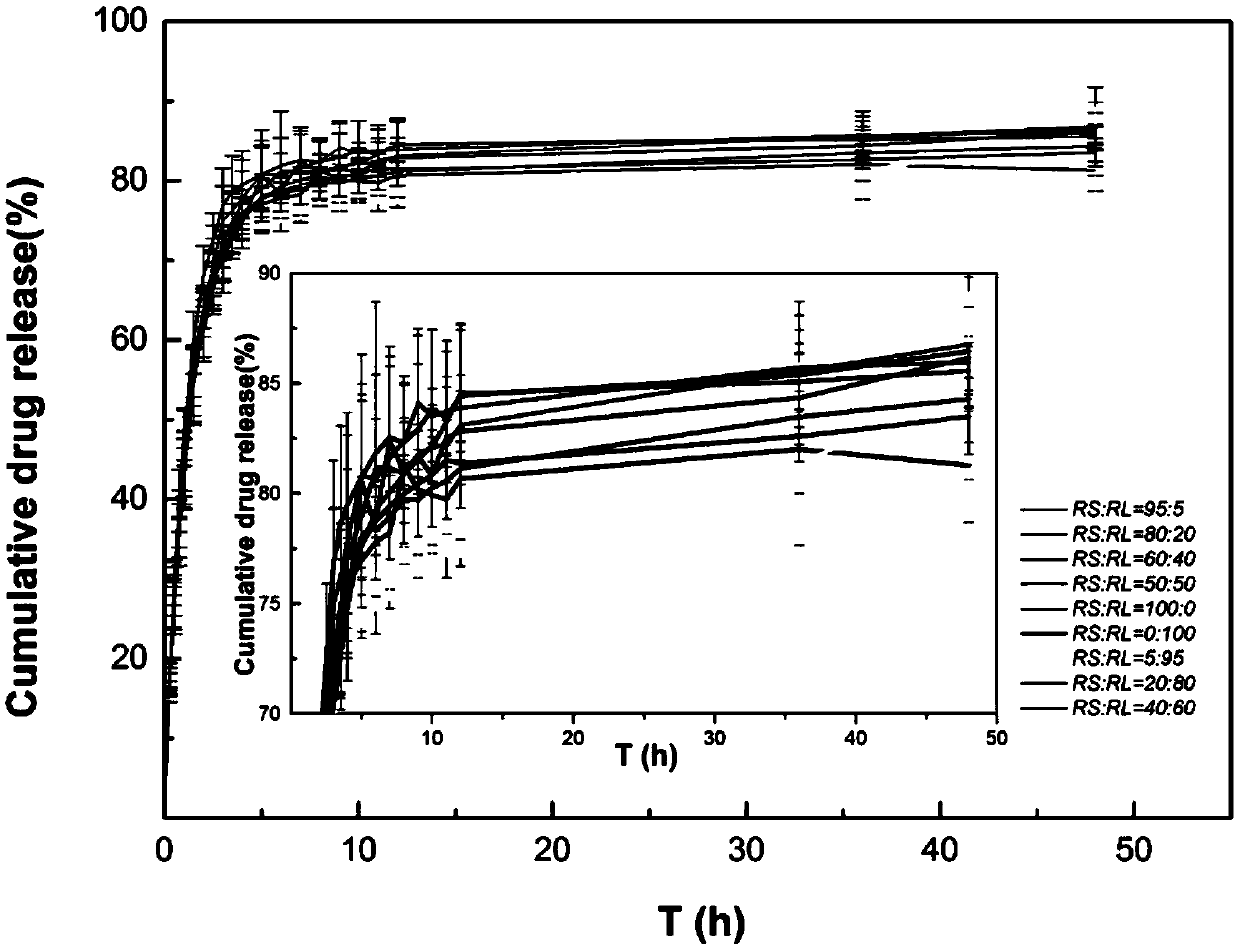
Preparation method for intraocular medicine delivery system of polyacrylic resin wrapping montmorillonite loading medicine
A technology of polyacrylic acid resin and drug delivery system, applied in the field of medicine, can solve the problems of drug liquid retention, low bioavailability, short-term, etc., and achieve the effects of improving absorption and utilization, simple and convenient operation, and small particle size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] (1) Place the montmorillonite in 5v / v% sulfuric acid, acidify it in a water bath at 70°C for 0.5h, wash it with double distilled water to neutrality, treat it ultrasonically to reduce the particle size, and obtain the acidified montmorillonite after drying. Acid-activated modified montmorillonite;
[0037] Prepare an aqueous solution of betaxolol hydrochloride (BH) with a concentration of 3g / L, add acidified montmorillonite whose weight is 1 / 3 of the weight of BH, adjust the pH to 4, absorb in a water bath at 50°C for 6h, centrifuge, and dry to obtain Montmorillonite-betaxolol hydrochloride compound (i.e. drug-loaded montmorillonite);
[0038] (2) Accurately weigh 425.6mg RL PO, 22.4mg Put RS PO, 56mg BH in a test tube, add 5mL of absolute ethanol, ultrasonically dissolve at 50°C, after it is completely dissolved, add 5mg of montmorillonite-betaxolol hydrochloride complex to obtain an organic phase;
[0039] Another 1% poloxamer 188 solution was taken as the water ...
Embodiment 2
[0043] (1) Place the montmorillonite in 5v / v% sulfuric acid, acidify it in a water bath at 70°C for 0.5h, wash it with double distilled water to neutrality, treat it ultrasonically to reduce the particle size, and obtain the acidified montmorillonite after drying. Acid-activated modified montmorillonite;
[0044] Prepare an aqueous solution of betaxolol hydrochloride (BH) with a concentration of 3g / L, add acidified montmorillonite whose weight is 1 / 3 of the weight of BH, adjust the pH to 4, absorb in a water bath at 50°C for 6h, centrifuge and dry, and prepare Demontmorillonite-betaxolol hydrochloride compound (i.e. drug-loaded montmorillonite);
[0045] (2) Accurately weigh 425.6mg RL PO, 22.4mg RS PO, 56mgBH in a test tube, add 5mL of acetone, ultrasonically dissolve at 50°C, after it is completely dissolved, add 5mg of montmorillonite-betaxolol hydrochloride complex to obtain an organic phase;
[0046] (3) Take another 1% poloxamer 188 solution as the water phase;
[...
Embodiment 3
[0049] (1) Place the montmorillonite in 5v / v% sulfuric acid, acidify it in a water bath at 70°C for 0.5h, wash it with double distilled water to neutrality, treat it ultrasonically to reduce the particle size, and obtain the acidified montmorillonite after drying. Acid-activated modified montmorillonite;
[0050] Prepare an aqueous solution of betaxolol hydrochloride (BH) with a concentration of 3g / L, add acidified montmorillonite whose weight is 1 / 3 of the weight of BH, adjust the pH to 4, absorb in a water bath at 50°C for 6h, centrifuge and dry, and prepare Demontmorillonite-betaxolol hydrochloride compound (i.e. drug-loaded montmorillonite);
[0051] (2) Accurately weigh 425.6mg RL PO, 22.4mg RS PO, 56mgBH in a test tube, add 5mL of dichloromethane, ultrasonically dissolve at 50°C, after it is completely dissolved, add 5mg of montmorillonite-betaxolol hydrochloride complex to obtain an organic phase;
[0052] Another 1% poloxamer 188 solution was taken as the water phas...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com