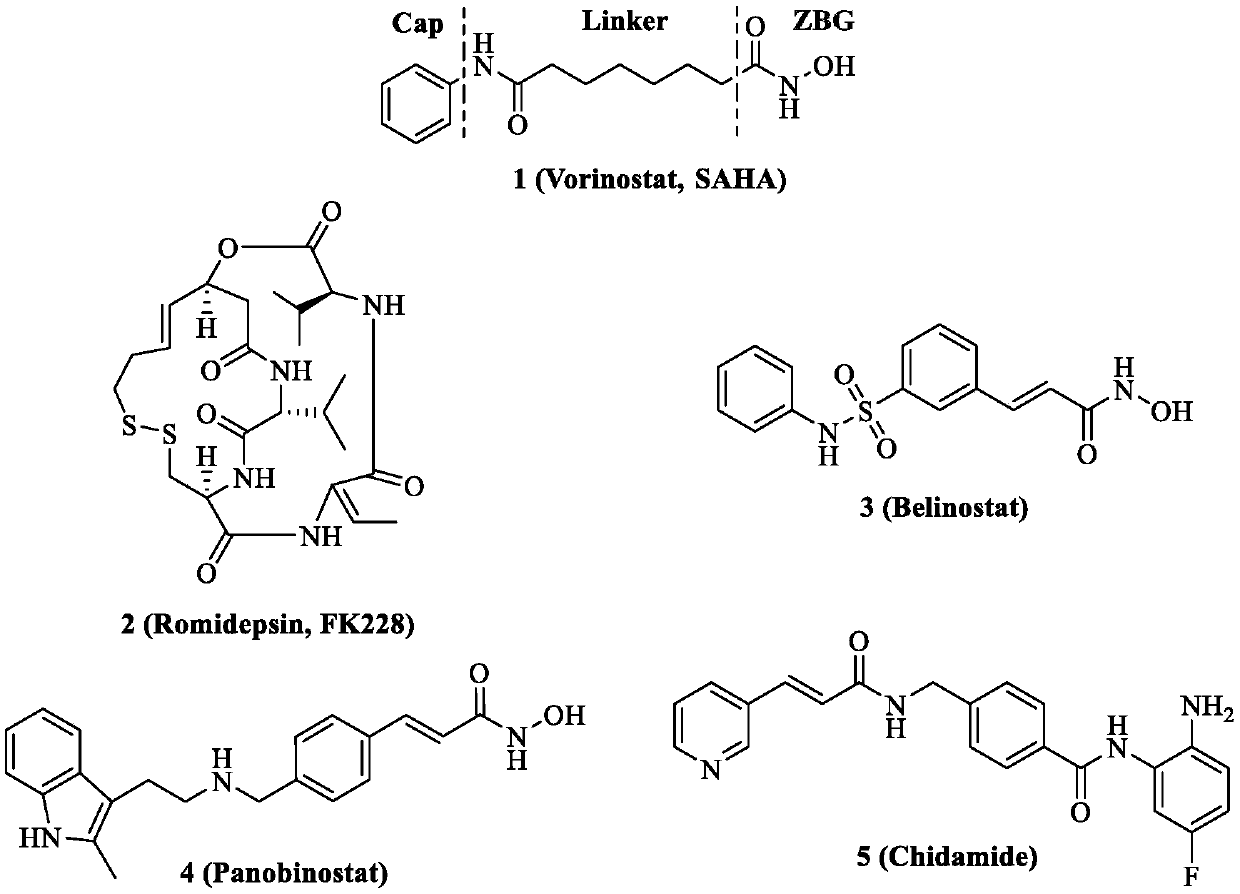
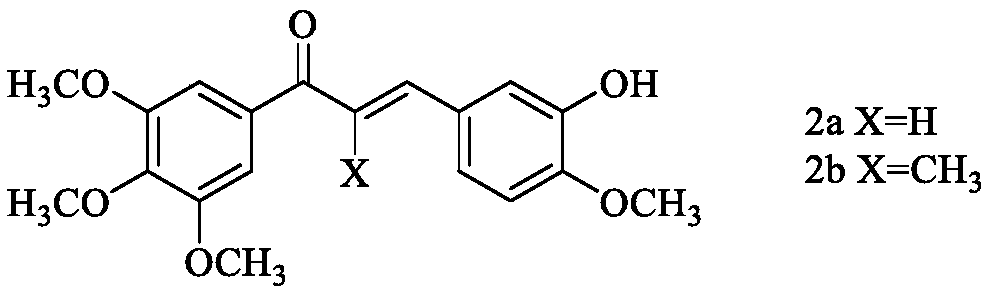
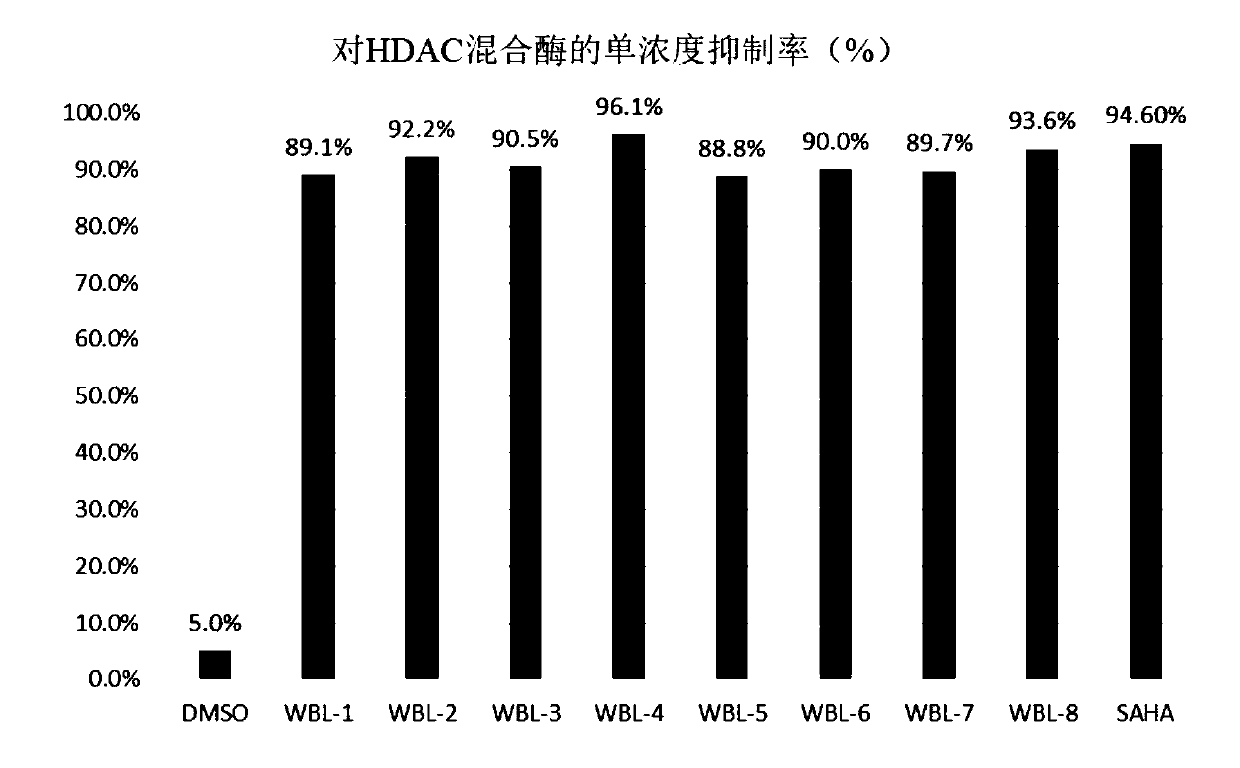
Histone deacetylase and microtubule dual target inhibitor and preparation method thereof
A deacetylase, dual-target technology, used in organic chemistry, drug combinations, anti-tumor drugs, etc., can solve the problem of few dual-target inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] The preparation of embodiment 1WBL-1
[0060] The structural formula of WBL-1 is:
[0061]
[0062] Its chemical name is: N-hydroxy-7-(2-methoxy-5-(3-keto-3-(3,4,5-trimethoxyphenyl)-1-(E)-propenyl ) phenoxy-heptanamide.
[0063] Its synthetic route is:
[0064]
[0065] The concrete steps of its synthetic method are as follows:
[0066] Step 1, Synthesis A: Weigh 3',4',5,-trimethoxyacetophenone (21.02g, 1mmol) and 3-hydroxy-4-methoxybenzaldehyde (15.22g, 1mmol) and dissolve in 200mL In methanol, 160 mL of 50% (W / V) sodium hydroxide solution was added to the above mixture, and the mixture was stirred overnight at room temperature under nitrogen protection. Distill methanol, and add 1M hydrochloric acid to adjust the pH value of the mixture to 1-2, extract three times with 20 ml of dichloromethane, collect the dichloromethane phase and dry the filtrate with anhydrous sodium sulfate, concentrate and purify by silica gel column chromatography (petroleum ether : e...
Embodiment 2
[0072] The preparation of embodiment 2WBL-2
[0073] The chemical formula of WBL-2 is:
[0074]
[0075] Its chemical name is: N-hydroxy-8-(2-methoxy-5-(3-keto-3-(3,4,5-trimethoxyphenyl)-1-(E)-propenyl ) phenoxy-octylamide.
[0076] Its synthetic route is:
[0077]
[0078] The concrete steps of its synthetic method are as follows:
[0079] Step 1, synthetic A: with embodiment 1 step 1;
[0080] Step 2. Synthesis of A8ES: The molar ratio of A (3.44g, 10mmol) to 8-bromooctanoic acid methyl ester is 1:1.1, the two are dissolved in 20mL of anhydrous N',N'dimethylformamide, and added twice The amount of anhydrous potassium carbonate, 60 ℃ oil bath overnight, TLC inspection reaction (petroleum ether: ethyl acetate = 2:1 (v / v)). Add water and extract with ethyl acetate, dry the ethyl acetate phase with anhydrous sodium sulfate, concentrate and purify by silica gel column chromatography (petroleum ether: ethyl acetate = 6:1) to obtain a yellow solid with a yield of 73%.
...
Embodiment 3
[0085] The preparation of embodiment 3WBL-3
[0086] The chemical formula of WBL-3 is:
[0087]
[0088] Its chemical name is: N-hydroxy-7-(2-methoxy-5-(2-methyl-3-keto-3-(3,4,5-trimethoxyphenyl)-1-( E)-propenyl)phenoxy-heptanamide.
[0089] Its synthetic route is:
[0090]
[0091] The concrete steps of its synthetic method are as follows:
[0092] Step 1, Synthesis B: Weigh 3', 4', 5'-trimethoxypropiophenone (15g, 66mmol) and 3-hydroxyl-4-methoxybenzaldehyde (33g, 66mmol) in equivalent molar amounts and dissolve in In methanol solvent, 11 mL of 50% (W / V) sodium hydroxide solution was added to the above mixture, and the mixture was stirred and reacted overnight under nitrogen protection, and the reaction was checked by TLC (petroleum ether: ethyl acetate=2:1 (v / v)). Add 1M hydrochloric acid to adjust the pH of the mixture to 1-2, and extract twice with 100 mL of dichloromethane. The dichloromethane phase was dried over anhydrous sodium sulfate, concentrated and t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com