Nitrogen-doped carbon quantum dot and preparation method thereof
A technology of carbon quantum dots and nitrogen doping, applied in the field of quantum dots, can solve the problems of high energy consumption, low quantum yield, and complicated preparation process, and achieve the effects of low energy consumption, stable fluorescence performance, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
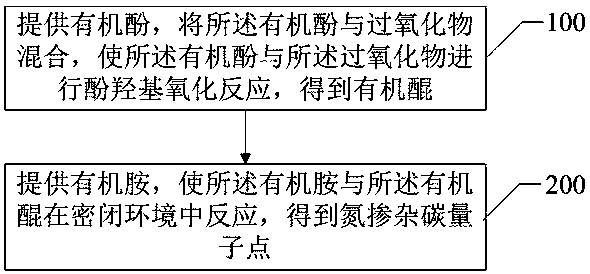
[0044] The preparation method of the nitrogen-doped carbon quantum dots of this embodiment comprises the following steps:
[0045] (1), phenolic hydroxyl oxidation reaction process
[0046] Add 1.28 g of phloroglucinol and 27 mL of deionized water into a 100 mL three-necked flask respectively, and stir at room temperature to obtain an aqueous solution of phloroglucinol. Next, 11 mL of hydrogen peroxide aqueous solution (30 wt%) was added to the above phloroglucinol aqueous solution, and stirred at room temperature for 10 min to obtain a uniform brown solution.
[0047] (2), Schiff base polycondensation and carbonization reaction process
[0048] Add 1.08 g of p-phenylenediamine to the brown solution in (1) above, stir evenly, then quickly transfer it to a 50 mL autoclave, and let it stand for 5 minutes to react, and finally a black reaction solution is obtained.
[0049] (3) Purification and collection process of nitrogen-doped carbon quantum dots
[0050] Take out the black ...
Embodiment 2
[0052] The preparation method of the nitrogen-doped carbon quantum dots of this embodiment comprises the following steps:
[0053] (1), phenolic hydroxyl oxidation reaction process
[0054] Add 1.28 g of phloroglucinol and 27 mL of deionized water into a 100 mL three-necked flask respectively, and stir at room temperature to obtain an aqueous solution of phloroglucinol. Next, 11 mL of hydrogen peroxide aqueous solution (30 wt%) was added to the above phloroglucinol aqueous solution, and stirred at room temperature for 10 min to obtain a uniform brown solution.
[0055] (2), Schiff base polycondensation and carbonization reaction process
[0056] Add 1.23 g of p-phenylenediamine to the brown solution in (1) above, stir evenly, then quickly transfer it to a 50 mL autoclave, let it stand for 5 min to react, and finally obtain a black reaction solution.
[0057] (3) Purification and collection process of nitrogen-doped carbon quantum dots
[0058] Take out the black reaction so...
Embodiment 3
[0060] The preparation method of the nitrogen-doped carbon quantum dots of this embodiment comprises the following steps:
[0061] (1), phenolic hydroxyl oxidation reaction process
[0062] Add 2.26 g of anthracenol and 27 mL of deionized water into a 100 mL three-necked flask respectively, and stir at room temperature to obtain an aqueous solution of anthracenol. Next, 11 mL of hydrogen peroxide aqueous solution (30 wt%) was added to the above-mentioned anthracenol aqueous solution, and stirred at room temperature for 10 min to obtain a uniform brown solution.
[0063] (2), Schiff base polycondensation and carbonization reaction process
[0064] Add 1.08 g of p-phenylenediamine to the brown solution in (1) above, stir evenly, then quickly transfer it to a 50 mL autoclave, and let it stand for 5 minutes to react, and finally a black reaction solution is obtained.
[0065] (3) Purification and collection process of nitrogen-doped carbon quantum dots
[0066] Take out the bla...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com