Synthesis and application of fluorescence-enhanced fluorescent molecule probe for rapidly detecting hydrogen sulfite ions or sulfite ions
A fluorescent molecular probe and bisulfite technology, applied in the field of biochemistry, can solve problems such as human toxicity and environmental problems, and achieve the effects of low detection limit, fast detection speed and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1: Preparation of Molecular Fluorescent Probe 1
[0026] 3-Ethyl-1,1,2-trimethyl-1H-benzo[e]indol-3-ium iodide 357 mg (0.97 mmol) and 2-(4-formylphenyl)phenanthroimidazole 300 mg (0.93 mmol) was dissolved in 10 ml of absolute ethanol, 2-3 drops of piperidine were added dropwise, and the reaction was stirred at room temperature for 8 hours. Then the reaction liquid was filtered, and the obtained solid was washed with absolute ethanol, and vacuum-dried to obtain 260 mg (42%) of the fluorescent molecular probe.
[0027] The proton nuclear magnetic spectrum data of above-mentioned gained probe: 1 H NMR (500 MHz, DMSO- d 6 ) δ 8.89 (s, 2H),8.62-8.65 (d, J = 16.4 Hz, 3H), 8.54 – 8.46 (m, 5H), 8.32-8.34 (d, J = 9.0Hz, 1H), 8.24-8.25 (d, J = 8.2 Hz, 1H), 8.16-8.18 (d, J = 8.9 Hz, 1H), 7.87 –7.60(m, 8H), 4.90-4.95 (q, J = 7.3 Hz, 2H), 2.09 (s, 6H), 1.58 (t, J = 7.3Hz, 3H).
Embodiment 2
[0028] Example 2: Preparation of Molecular Fluorescent Probe 1
[0029] 3-Ethyl-1,1,2-trimethyl-1H-benzo[e]indol-3-ium iodide 357 mg (0.97 mmol) and 2-(4-formylphenyl)phenanthroimidazole 300 mg (0.93 mmol) was dissolved in 10 ml of absolute ethanol, 2-3 drops of pyrrolidine were added dropwise, and the reaction was stirred at room temperature for 8 hours. Then the reaction solution was filtered, and the obtained solid was washed with absolute ethanol, and vacuum-dried to obtain 250 mg (40%) of the fluorescent molecular probe.
[0030] The proton nuclear magnetic spectrum data of above-mentioned gained probe: 1 H NMR (500 MHz, DMSO- d 6 ) δ 8.89 (s, 2H),8.62-8.65 (d, J = 16.4 Hz, 3H), 8.54 – 8.46 (m, 5H), 8.32-8.34 (d, J = 9.0Hz, 1H), 8.24-8.25 (d, J = 8.2 Hz, 1H), 8.16-8.18 (d, J = 8.9 Hz, 1H), 7.87 –7.60(m, 8H), 4.90-4.95 (q, J = 7.3 Hz, 2H), 2.09 (s, 6H), 1.58 (t, J = 7.3Hz, 3H).
Embodiment 3
[0031]Example 3: Application of fluorescence-enhanced fluorescent molecular probes for rapid detection of bisulfite ions or sulfite ions.
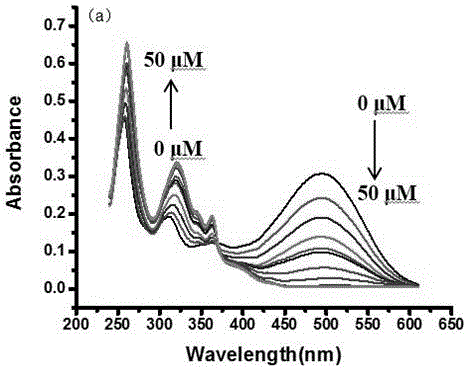
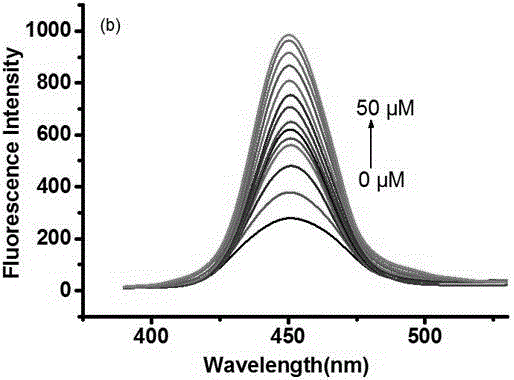
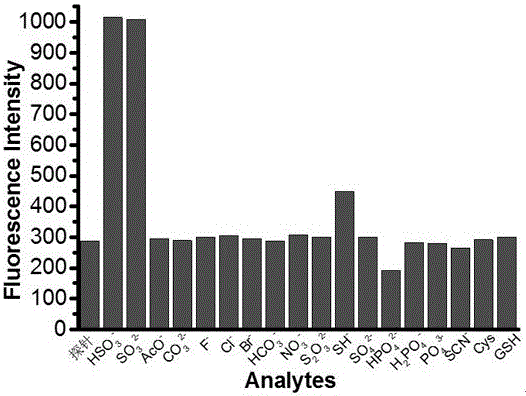
[0032] Dissolve the molecular fluorescent probe in dimethyl sulfoxide (DMSO) to make a 1 mmol / L probe solution; add the corresponding ethanol and PBS (pH=7.4) buffer to the probe solution to make a 10 µM (organic phase: PBS aqueous phase = 4:6, v / v) solution, test its ultraviolet absorption spectrum and fluorescence emission spectrum changes. In the ultraviolet absorption spectrum and fluorescence emission spectrum, the probe has high selectivity to bisulfite ion or sulfite ion. With the increase of the concentration of bisulfite ion or sulfite ion, its ultraviolet absorption The spectrum changes obviously, the absorption peak at the wavelength of 493 nm gradually decreases, and the absorption peak at the wavelength of 319 nm gradually increases, and the obvious color change from red to colorless is also suitable for naked eye detection, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com