Lithium tetrafluorooxalatophosphate preparation method
A technology of lithium tetrafluorooxalate phosphate and lithium oxalate, which is applied in chemical instruments and methods, compounds of group 5/15 elements of the periodic table, organic chemistry, etc., can solve problems such as adverse effects on battery performance and product quality degradation, and achieve Overcome the effect of large reaction consumption, short reaction time and improvement of product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
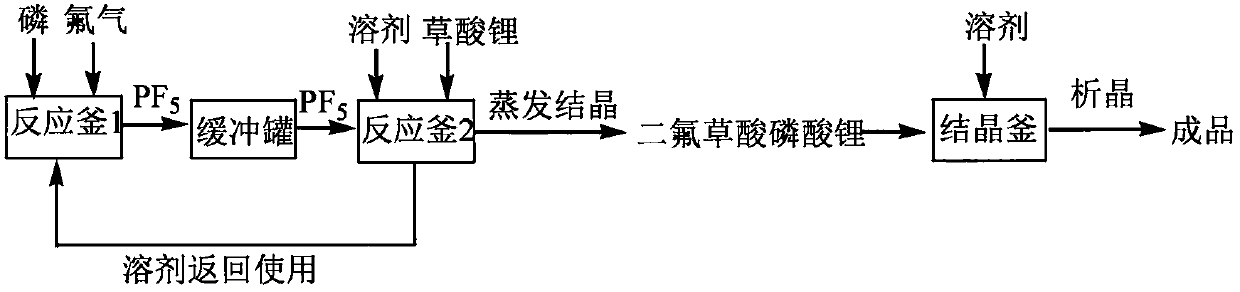
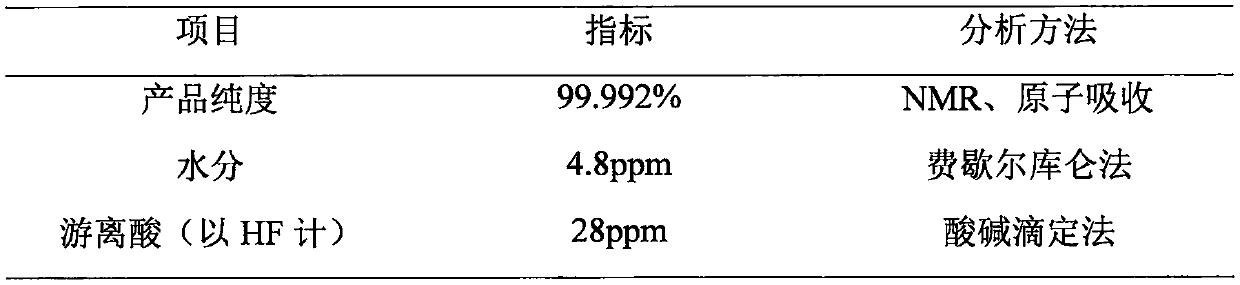
[0034] At room temperature, 101.9 g of lithium oxalate and 800 g of DMC were added to the jacketed reaction vessel 2 and stirred for 1 hour to form a uniform suspension of lithium oxalate and DMC. Add 65.17g of red phosphorus to the reaction vessel 1 in advance, then slowly pass in 200g of fluorine gas, pass a low-temperature cooling medium into the jacket to control the temperature, and then pass the phosphorus pentafluoride produced in the process into the reaction vessel 2 , The gas outlet rate is controlled to 2L / Min. During the process, always pay attention to temperature changes, and control the temperature below 30°C. After the gas is introduced, continue to stir for 4 hours. After filtration, after concentration and crystallization, 174.43 g of product was obtained, of which the theoretical output was 202 g, and the product yield reached 86.35%. The product purity of the obtained product is 99.96%, the water content is 4.8 ppm, and the acid content is 28 ppm.
[0035] Ta...
Embodiment 2
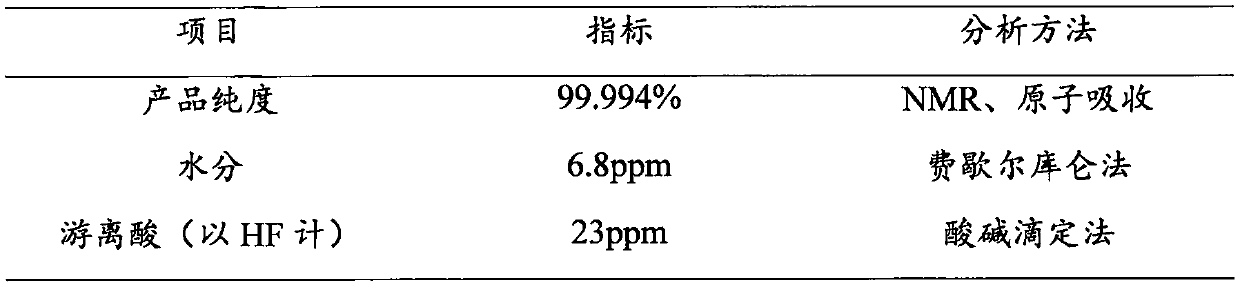
[0038] At room temperature, 110.2 g of lithium oxalate and 850 g of DMC were added to a jacketed reaction vessel 2 and stirred for 1 hour to form a uniform suspension of lithium oxalate and DMC. Into the reaction vessel 1, 72g of red phosphorus was introduced in advance, and then 200g of fluorine gas was slowly introduced, and a low-temperature cooling medium was introduced into the jacket to control the temperature, and then the phosphorus pentafluoride produced in the process was introduced into the reaction vessel 2 In the process, the gas outlet rate is controlled to 2L / Min. During the process, pay attention to temperature changes at all times, and control the temperature below 30°C. After the gas is introduced, continue to stir for 4 hours. After filtration, after concentration and crystallization, 175.23g of product was obtained, of which the theoretical output was 210g, and the product yield reached 86.74%. The product purity of the obtained product is 99.8%, the water c...
Embodiment 3
[0042] At room temperature, 105 g of lithium oxalate and 820 g of DMC were added to the jacketed reaction vessel 2 and stirred for 1 hour to form a uniform suspension of lithium oxalate and DMC. Add 68g of white phosphorus to the reaction vessel 1 in advance, and then slowly pass in 200g of fluorine gas, pass a low-temperature cooling medium into the jacket to control the temperature, and then pass the phosphorus pentafluoride produced in the process into the reaction vessel 2. The export rate is controlled to 2L / Min. During the process, keep an eye on the temperature change, and control the temperature below 30°C. After the gas is introduced, continue to stir for 4 hours. After filtration, after concentration and crystallization, 174.52 g of product was obtained, of which the theoretical output was 205 g, and the product yield reached 86.4%. The product purity of the obtained product is 99.90%, the water content is 5.2 ppm, and the acid content is 20 ppm.
[0043] Table Three
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| water content | aaaaa | aaaaa |
| water content | aaaaa | aaaaa |
| water content | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com