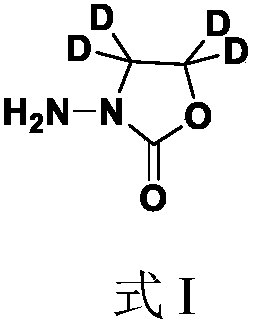
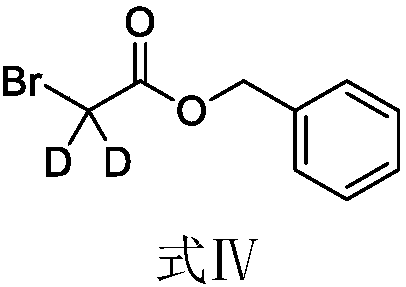
Method for synthesizing nitrofuran metabolite furazolidone AOZ-D4
A technology of AOZ-D4 and furazolidone, which is applied in the fields of organic chemistry and organic chemistry, can solve the problems that there are no literatures and patents on the synthesis of furazolidone, and achieve the effect of cheap synthetic raw materials, high yield and short synthesis cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1, Furazolidone AOZ-D4
[0042] Step 1: Synthesis of Deuterobromoacetic Acid
[0043] Add 3.7ml TFAA (trifluoroacetic anhydride, 46.8mmol) to 1.0mL CH3COOH-D4, add 0.85mL bromine (17.2mmol), react at room temperature for 16h, after the reaction, add deuterium water to quench, reduce pressure Evaporate to dryness to obtain deuterobromoacetic acid.
[0044] The second step: synthesis of deuterated benzyl acetate
[0045] Mix 1.0mL deuterobromoacetic acid (15.6mmol), 15.6mL oxalyl chloride (31.2mmol, 2.0M) and 0.01ml DMF (trace amount), react at 60°C for 3h, then add 4.86ml benzyl alcohol (46.8mmol), Reaction at room temperature for 16h, with D 2 O quenching affords benzyl deuterated acetate.
[0046] The third step: Synthesis of deuterated bromoethanol
[0047] 1.2g NaBD 4 (31.2mmol) was dissolved in 40mL THF, deuterated benzyl acetate (15.6mmol) was added dropwise to the reaction solution, reacted at room temperature for 6h, and evaporated to dryness und...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com