Injectable and degradable artificial bone material and preparation method thereof
A technology of artificial bone and main material, applied in the field of biomedical materials, can solve the problems of poor pore structure, incomplete degradation, low biological activity, etc., to reduce the risk of infection, adjust the porosity, and improve the effect of biological activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The injectable self-curing degradable bone filling material of this embodiment consists of two parts, a solid phase and a liquid phase, and its preparation method is as follows:
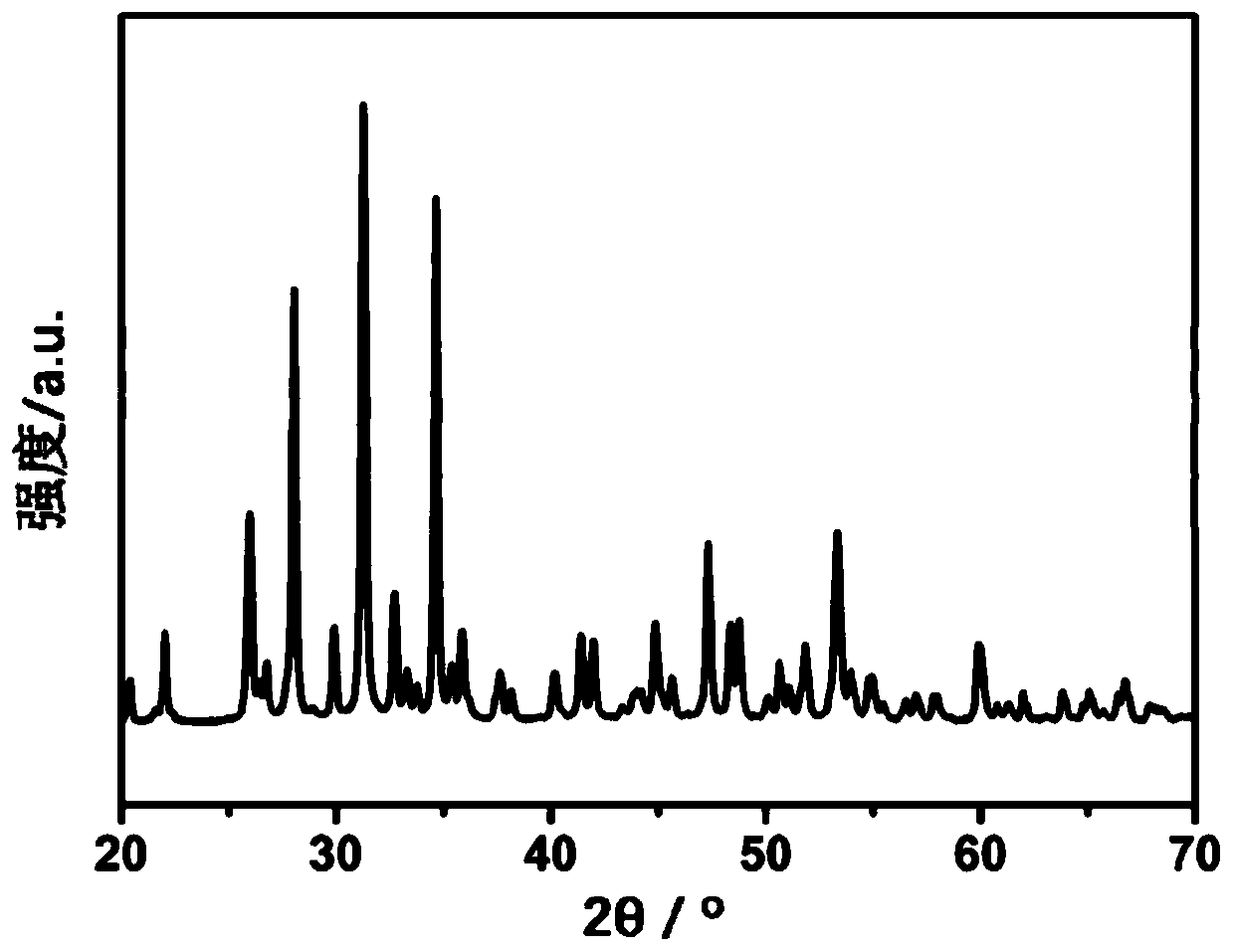
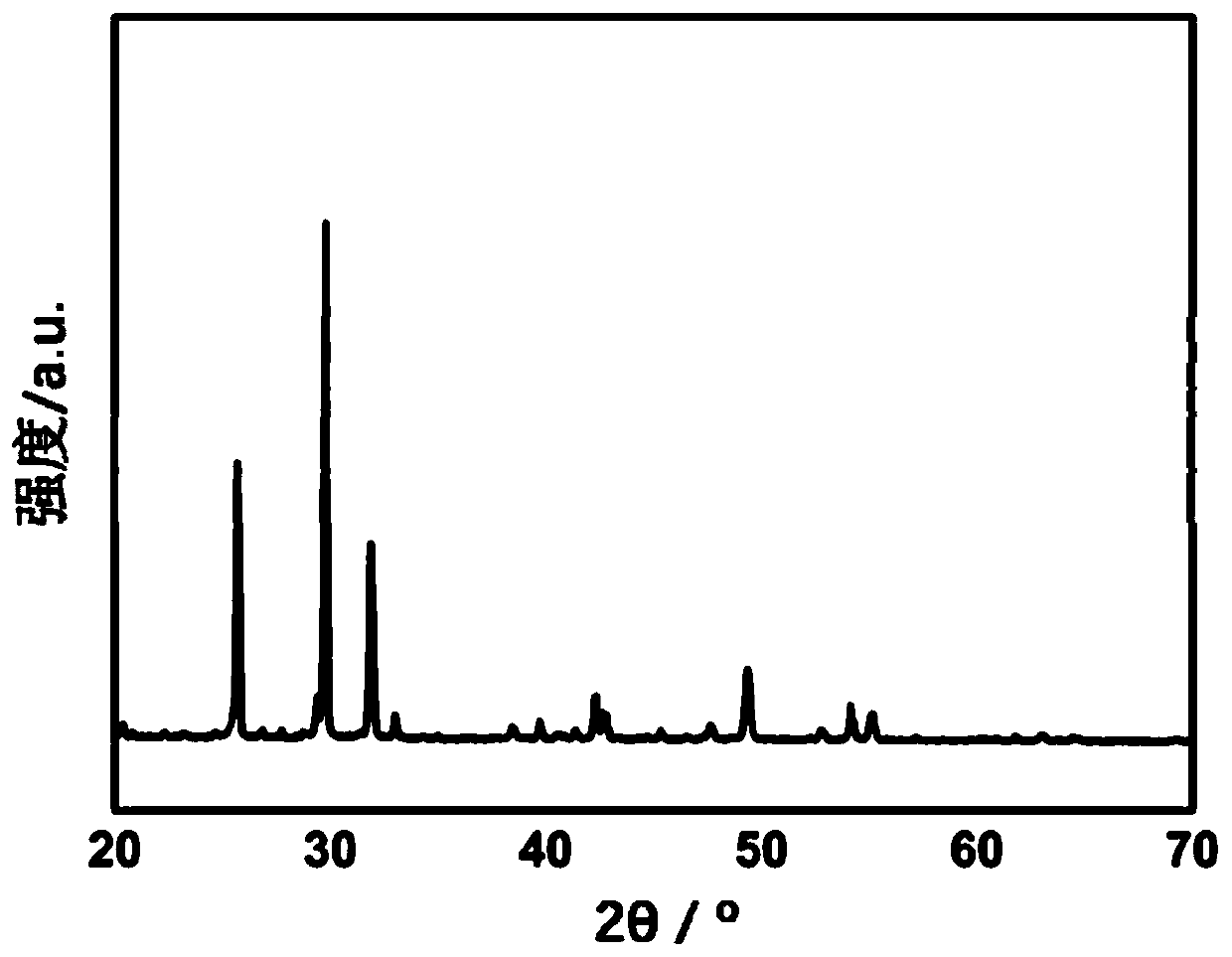
[0034] First take by weighing 0.9g of β-TCP (Mg / (Ca+Mg)=10at.%) material ( figure 1 ) and 2.1g of CSH materials with a particle size of 5-60μm ( figure 2 ), and the two materials are fully mixed to obtain a solid phase material. Add 1.2ml of PVA aqueous solution (concentration: 5wt%, liquid phase) to the above-mentioned solid phase powder, and mix uniformly for 30 seconds to prepare an injectable and degradable artificial bone material (30%β-TCP+70%CSH).
[0035] The curing time is measured with reference to the ASTM C191-18a standard. The solid-liquid mixed paste material is injected into a φ6*10mm stainless steel mold through a syringe, and then placed in a mold box (temperature: 37°C, humidity: 95%), and samples are taken out regularly Carry out the Vicat meter measurement until the Vica...
Embodiment 2
[0038] The injectable self-curing degradable bone filling material of this embodiment consists of two parts, a solid phase and a liquid phase, and its preparation method is as follows:
[0039] First weigh 0.87g of β-TCP material (Mg / (Ca+Mg)=10at.%) with an average particle size of 10-40μm after soft agglomeration, 2.1g of CSH material with an average particle size of 5-60μm and 0.03g metal Magnesium powder (particle size less than 75 μm), the three are fully mixed to obtain a solid phase material. Add 1.2ml of PVA aqueous solution (concentration 5wt%, liquid phase) to the above-mentioned solid phase powder, and make an injectable and degradable artificial bone material after uniform mixing for 30 seconds
[0040] (29% β-TCP + 70% CSH + 1% Mg).
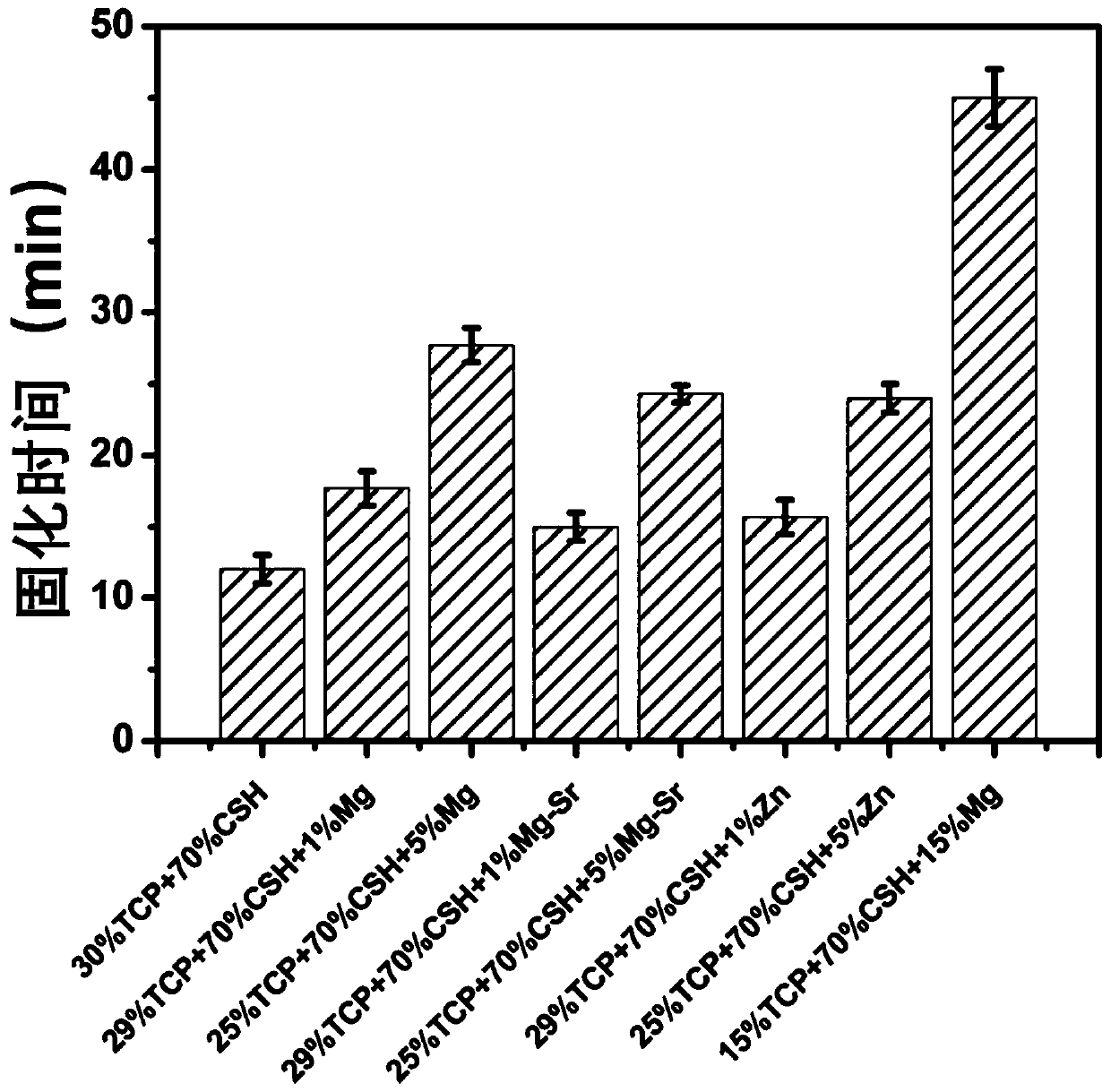
[0041] With reference to the ASTM C191-18a standard, the final setting time of the sample measured is 17.7 ± 1.2min ( image 3 ). The injectable artificial bone material was prepared into φ6*10mm samples (5 parallel samples), and t...
Embodiment 3
[0043] The injectable self-curing degradable bone filling material of this embodiment consists of two parts, a solid phase and a liquid phase, and its preparation method is as follows:
[0044] First weigh 0.75g of β-TCP material (Mg / (Ca+Mg)=10at.%) with an average particle size of 10-40μm after soft agglomeration, 2.1g of CSH material with an average particle size of 5-60μm and 0.15g metal Magnesium powder (less than 75 μm), the three are fully mixed to obtain a solid phase material. Add 1.2ml of PVA aqueous solution (concentration 5wt%, liquid phase) in above-mentioned solid phase powder, make injectable, biodegradable artificial bone material (25%β-TCP+70%CSH+5%Mg ).
[0045] With reference to the ASTM C191-18a standard, the final setting time of the sample measured is 27.7 ± 1.2min ( image 3 ), the injectable artificial bone material was prepared into φ6*10mm samples (5 parallel samples), and the compressive strength of the samples measured after complete curing was 2.2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com